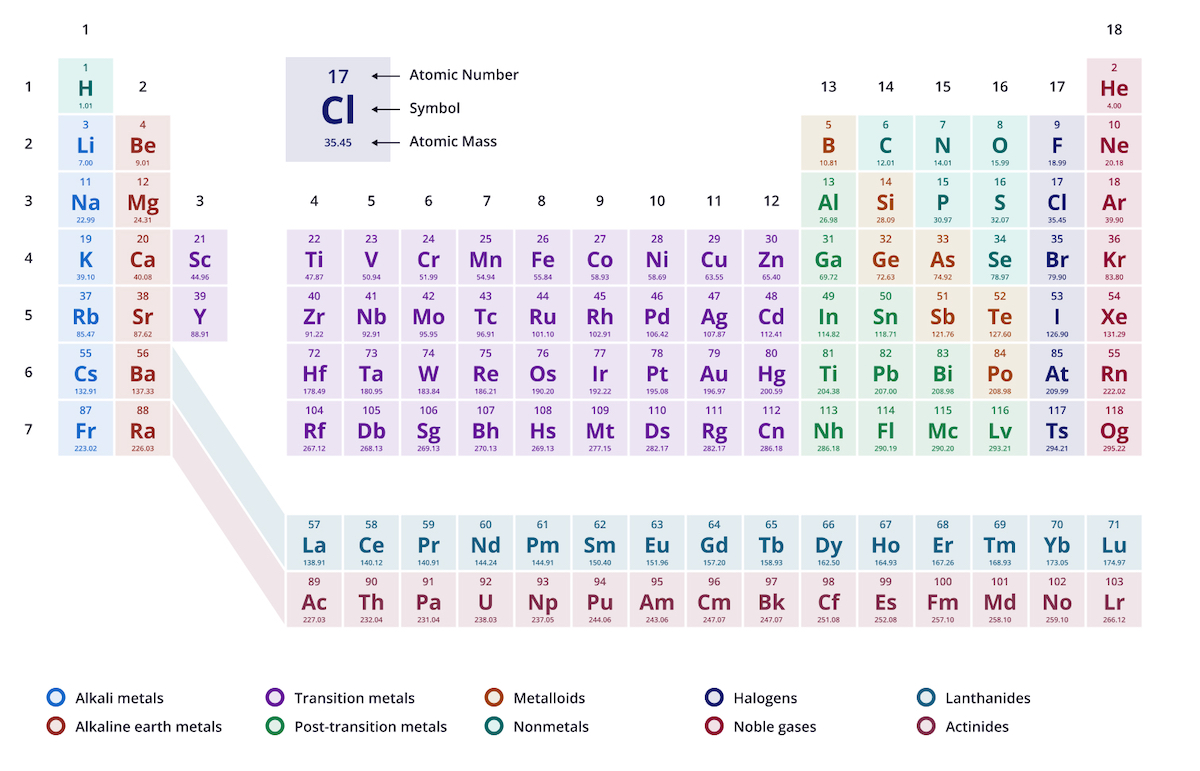
Chemistry is basically the study of everything. Yet, most of us look at the periodic table and see a terrifying grid of letters that looks like a failed game of Tetris. If you’ve ever tried a periodic table of elements quiz, you know that sinking feeling when you can't remember if "K" is Krypton or Potassium. (Spoiler: It’s Potassium, because of the Latin kalium). It's weird. We spend years in school staring at this chart, but the second we’re asked to name a halogen or find an alkali metal without looking, our brains go blank.
Honestly, the table is a masterpiece of organization, but it’s taught in the most boring way possible. We memorize the symbols. We forget them two days later. Most people treat a chemistry quiz like a chore instead of a map of the universe. But if you actually understand the logic—the "why" behind the rows and columns—you don’t have to memorize much at all.
The Logic Behind the Grid (And Why Quizzes Love It)
The periodic table isn't just a list. It’s a literal cheat sheet. Dmitri Mendeleev, the guy who famously "dreamed" the structure, was so confident in his patterns that he left gaps for elements that hadn't even been discovered yet. He knew they had to exist because the math demanded it. When you take a periodic table of elements quiz, the questions usually focus on these patterns.
Rows are called periods. Columns are groups.
👉 See also: Why Tom Ford Body Shimmer Is Still the Only Glow That Actually Matters
The elements in a column are basically siblings. They act alike. Group 18? Those are the Noble Gases. They’re the "cool kids" of the table—completely stable, rarely reacting with anyone else because their outer shells are full. Then you have Group 1, the Alkali Metals. They are the exact opposite. They are desperate to get rid of one electron, which makes them incredibly reactive. Throw a chunk of pure Sodium (Na) into water, and it doesn't just sink; it explodes. That’s a classic quiz question right there.
Most people fail quizzes because they focus on the names instead of the properties. If you know that Fluorine is at the top right (ignoring the noble gases), you know it’s the most electronegative element. It’s a "bully." It wants electrons more than anyone else. Once you grasp that, you don't need to memorize a table of values; you just need to know the geography.
The Symbols That Trip Everyone Up
Why is Lead "Pb"? Why is Gold "Au"?
This is where a periodic table of elements quiz usually gets mean. It’s easy to remember that Oxygen is "O" or Carbon is "C." But the ancient elements—the ones humans have used for thousands of years—use their Latin names.
- Lead (Pb): From Plumbum. It’s why we call it "plumbing."
- Gold (Au): From Aurum, meaning "shining dawn."
- Mercury (Hg): From Hydrargyrum, which translates to "liquid silver."
- Iron (Fe): From Ferrum.
If you're studying for a test, stop looking at the English names. Look at the Latin roots. It’s a lot easier to remember Tungsten is "W" if you know it’s named after Wolfram, a mineral found in Germany.
Transitions and the "F-Block" Mess
The middle of the table—the Transition Metals—is where things get messy. This is the D-block. These elements are the workhorses of our world. Iron, Copper, Silver, Zinc. They’re predictable in some ways but weird in others. And then there are those two rows floating at the bottom: the Lanthanides and Actinides.
👉 See also: Why The Rabbit Who Wants to Fall Asleep Still Divides Parents
Why are they down there? Space. Seriously. If you put them where they actually belong (between groups 3 and 4), the table would be so wide it wouldn't fit on a standard piece of paper. Most periodic table of elements quizzes won't grill you on the obscure properties of Lutetium or Lawrencium, but they will ask you why they’re separated. Now you know. It’s just graphic design.
How to Actually Pass Your Next Chemistry Test
If you want to ace a periodic table of elements quiz, you need a strategy that isn't just "stare at the wall and cry."
- Focus on the "Big Six": Carbon, Hydrogen, Nitrogen, Oxygen, Phosphorus, and Sulfur. (CHNOPS). These are the building blocks of life. If you know these, you know biology.
- Master the Diatomics: Some elements hate being alone. They always travel in pairs. Have No Fear Of Ice Cold Beer (Hydrogen, Nitrogen, Fluorine, Oxygen, Iodine, Chlorine, Bromine). This is a top-tier mnemonic.
- Trends are King: Atomic radius gets smaller as you go left to right (because the nucleus pulls harder on the electrons). It gets bigger as you go down (because you’re adding "shells"). If a quiz asks which atom is bigger, Cesium or Helium, you don't need to look up a chart. You just need to know their position.
Why Does This Even Matter?
You might think you’ll never use this. You’re wrong. Understanding the periodic table helps you understand the news, your health, and the tech in your pocket.
👉 See also: Rye Chocolate Chip Cookies Are Actually Better Than The Original
Ever wonder why your phone uses a Lithium-ion battery? Look at Lithium on the table. It’s tiny. It’s the lightest metal. It’s in Group 1, meaning it’s super eager to move electrons around. That’s literally what a battery does. If we tried to make a "Lead-ion" battery for a phone, your pocket would weigh five pounds.
What about the "rare earth metals" everyone talks about in the news regarding China and trade? Those are the Lanthanides we mentioned earlier. They aren't actually that "rare" in the dirt, but they are incredibly difficult to separate from each other. Neodymium is what makes the magnets in your headphones work. Without the f-block, your Spotify wouldn't sound nearly as good.
Misconceptions You Should Probably Forget
There’s a common myth that the periodic table is "finished." It’s not. In 2016, we added four new elements: Nihonium, Moscovium, Tennessine, and Oganesson. These are man-made. They only exist for a fraction of a second in a lab before they decay into something else.
Also, don't assume the table looks the same everywhere. Different countries use different layouts. Some chemists prefer a "spiral" version or a "3D flower" version because it better represents how the orbitals actually work. The grid we use is just the most "user-friendly" version.
Actionable Next Steps
To actually get good at a periodic table of elements quiz, stop trying to memorize the whole thing at once. It's a waste of time. Instead:
- Download an interactive app: Look for one that lets you click on the element to see its real-world use. Seeing a picture of a banana for Potassium helps it stick.
- Learn the groups first: Don't learn element 1 through 118. Learn the Alkali Metals. Then learn the Halogens. Then the Noble Gases. Categorization is how the human brain stores data.
- Practice with "Blank Table" drills: Print out a grid with no letters and try to fill in just the first 20 elements. Once you have the foundation, the rest of the "heavy" metals feel less intimidating.
- Connect it to the real world: Next time you’re reading a food label and see "Sodium Phosphate," go find those two on the table. Seeing how they sit on opposite sides of the chart explains why they bond so tightly together.
The periodic table isn't a list of things to memorize for a grade; it's the ingredient list for the entire universe. Treat it like a puzzle, and suddenly those quizzes aren't so scary anymore.