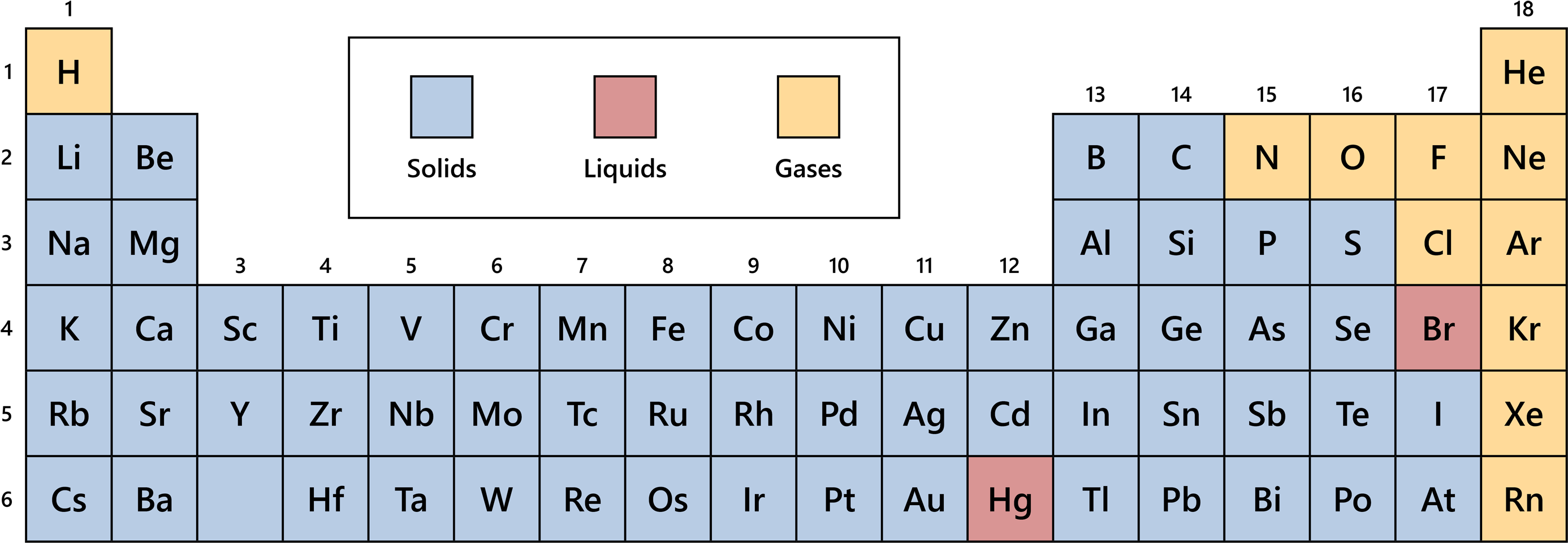
Most of us remember that colorful poster hanging on the wall of our high school chemistry class. It looked organized. Neat. It gave off the impression that nature has a tidy filing system. But when you start looking at the solid liquid gas on periodic table layouts, you realize the universe is actually a bit of a mess.
Temperature is the big boss here.
If you change the room temperature by just a few degrees, the entire map of the periodic table starts to shift. We usually talk about "standard conditions"—basically a comfortable room at 25°C (77°F). At that specific temperature, the world is mostly solid. It's a heavy, metallic world. But the exceptions? Those are where the real stories are.
🔗 Read more: How Much Is Sling TV a Month? Why the Answer Isn’t Just One Number
The Weirdly Short List of Liquids
It’s actually kind of wild how rare liquids are. Out of 118 identified elements, only two are liquid at standard room temperature. Just two.
Mercury and Bromine.
Mercury is the one everyone knows—that silver, "quicksilver" stuff that looks like it belongs in a sci-fi movie. It’s a metal, but it refuses to stay rigid. Why? Because its electrons are moving so fast (relativistic effects, if we’re being nerdy) that they don't want to share or bond with other mercury atoms. They’re loners.
Then you’ve got Bromine. It’s a halogen. It’s a nasty, reddish-brown liquid that fumes like crazy. Honestly, it’s one of the most unpleasant things to work with in a lab. It’s heavy, it’s volatile, and it’ll eat through your gloves if you aren't careful.
But here is where it gets interesting. If the room gets just a little bit warmer—say, a hot summer day in Arizona—the "liquid club" grows. Cesium, Gallium, and Francium are right on the edge. Gallium is the most famous prank metal. It melts at about 29.7°C. That means if you hold a chunk of solid gallium in your hand, it literally turns into a silver puddle just from your body heat. It’s a solid on the table, but a liquid in your palm. This fluidity shows that the solid liquid gas on periodic table distinctions are way more fragile than the posters suggest.
The Gas Giants of the Microscopic World
Gases are the social distancing champions of the elements. While solids are packed tight like a morning commute on the subway, gas atoms are flying around with massive amounts of empty space between them.
On the periodic table, the gases are clustered mostly on the right side. You have the "Noble Gases" in Group 18—Helium, Neon, Argon, Krypton, Xenon, and Radon. They are the snobs of the chemical world. They have full electron shells, so they don't feel the need to react with anyone else. They just float.
Then you have the "diatomic" gases. Hydrogen, Nitrogen, Oxygen, Fluorine, and Chlorine. These guys don't like being alone. They travel in pairs ($H_2$, $O_2$, etc.).
📖 Related: Why the Panasonic NCR18650B Maximum Continuous Discharge Current Still Catches People Off Guard
Hydrogen is the weirdo here. It’s sitting way over on the top left, usually floating above the alkali metals. It’s a gas, but it’s in a column of highly reactive solids. Some scientists, like those at Harvard who have been experimenting with high-pressure physics, have actually managed to turn hydrogen into a metal. Imagine that: metallic hydrogen. It’s thought to exist in the cores of Jupiter and Saturn. In those environments, the solid liquid gas on periodic table rules we learn in school are completely tossed out the window.
Solids: The Heavy Majority
The vast majority of the periodic table is solid. Carbon, Gold, Iron, Silicon—these are the building blocks of our physical reality.
But even "solid" is a broad term. You have the soft solids, like Sodium, which you can cut with a butter knife. It feels more like cold wax than what we typically think of as "metal." Then you have the ultra-hard solids like Diamond (a form of Carbon) or Tungsten, which has the highest melting point of all elements at a staggering 3422°C.
What Determines the State?
It all comes down to the tug-of-war between kinetic energy and inter-atomic forces.
- Kinetic Energy: This is the "jiggle." The hotter things are, the more atoms want to fly apart.
- Attraction: This is the "glue." Electrostatic forces want to pull atoms together.
When the glue is stronger than the jiggle, you get a solid. When they are roughly equal, you get the flowing chaos of a liquid. When the jiggle wins, you get a gas.
The Synthetic Mystery Zone
As you move toward the bottom of the periodic table, things get spooky. We are talking about elements like Oganesson (118) or Tennessine (117). These don't exist in nature. We create them in particle accelerators for fractions of a second.
Because they decay so fast, we often don't even know if they are solid, liquid, or gas. We have to guess based on trends. For a long time, people assumed Oganesson would be a gas because it’s in the Noble Gas column. But recent relativistic calculations suggest that because its atoms are so heavy and its electrons are moving so strangely, it might actually be a solid at room temperature.
This is a huge deal in chemistry circles. It means the "periodicity" (the predictable patterns) of the table starts to break down once the nuclei get too big. Nature starts behaving differently when things get that heavy.
Practical Insights for the Real World
Understanding the states of elements isn't just for passing a test. It’s the reason your phone works.
Silicon is a solid, but it’s a "semiconductor." Its state and its ability to hold onto electrons allow us to etch billions of tiny switches onto a chip. If Silicon were a gas at room temperature, the digital age wouldn't exist.
If you are looking at the solid liquid gas on periodic table for practical applications, keep these nuances in mind:
- Thermal Expansion: Solids aren't perfectly static. They expand. This is why bridges have "teeth" (expansion joints). If you use a metal with a high expansion rate in a precision instrument, it'll fail as the temperature swings.
- Vapor Pressure: Even solids "off-gas." This is why you can smell a piece of copper or a lead pipe. A tiny bit of that solid is escaping into the air.
- The Triple Point: Every element has a specific temperature and pressure where it can exist as a solid, liquid, and gas all at the same time. For water, it’s just above freezing at very low pressure. For elements like Carbon, reaching a liquid state is incredibly difficult and requires specialized equipment.
Changing Your Perspective
Don't look at the periodic table as a finished map. Look at it as a snapshot.
If we lived on a planet where the average temperature was 500°C, the "liquids" and "gases" would dominate the left side of the table too. Our definition of what is "normal" is entirely based on the thin crust of Earth.
If you're trying to memorize these for a project or a hobby in metallurgy or 3D printing, focus on the "p-block" (the right side). That’s where all the action is. That’s where the state changes happen most frequently and where the most interesting chemical reactions occur.
Next Steps for Deeper Mastery
To truly get a handle on how these states affect the world around you, your next move should be to look at Phase Diagrams. A phase diagram shows you exactly when an element flips from solid to liquid based on pressure.
You can also look into the "Island of Stability" theory. This is the idea that eventually, we might find super-heavy elements that don't decay in milliseconds—elements that could stay solid and usable for years, potentially opening up brand new types of materials we can't even imagine yet.
Start by picking one element, like Iodine, and look at its sublimation—how it skips the liquid phase entirely and goes straight from solid to purple gas. Seeing it in action makes the theory stick way better than any textbook ever could.