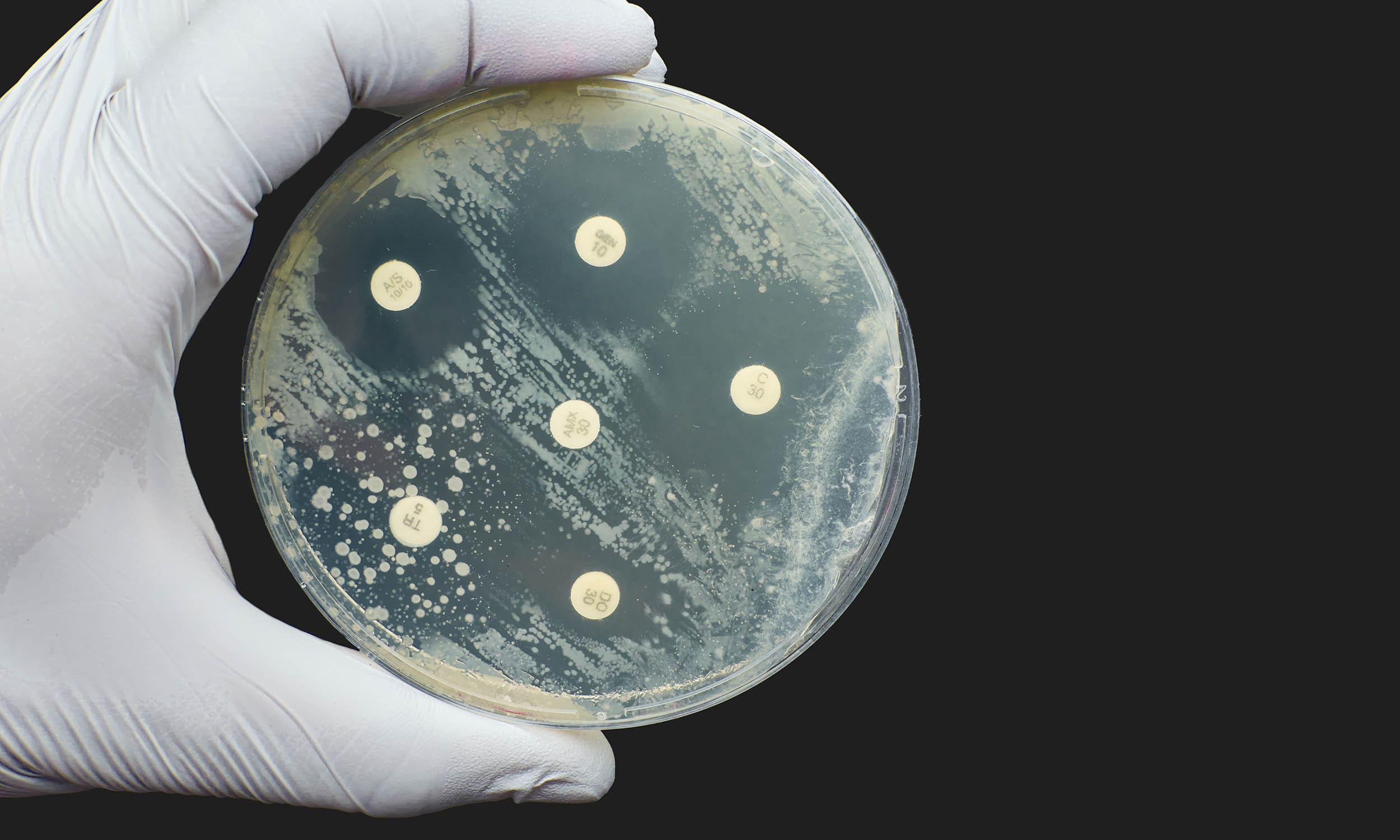
You've seen it. That clear, ghost-like circle surrounding a white paper disc on a carpet of bacteria. It looks like a tiny biological "no man's land." In science classrooms and professional labs alike, the disinfectants in zone of inhibition biology experiment is a rite of passage. It’s the Kirby-Bauer method’s more domestic cousin. We take everyday household cleaners—bleach, Lysol, maybe some "natural" tea tree oil—and pit them against microbes like E. coli or Staphylococcus epidermidis.
But here’s the thing. Most people do it wrong.
They assume a bigger circle means a "better" disinfectant. That is a massive misconception. If you’re looking at a 20mm zone for ethanol and a 10mm zone for bleach, you might think the booze wins. You’d be wrong. Biology is messy, and the physics of how chemicals move through agar is even messier.
The Physics of the "Kill Zone"
Let's talk about diffusion. When you place a saturated disc on an agar plate, the disinfectant doesn't just sit there. It starts a slow, outward crawl. This is governed by Fick’s Laws of Diffusion. Essentially, the chemical moves from an area of high concentration (the disc) to low concentration (the surrounding agar).
The "Zone of Inhibition" is the space where the concentration of that disinfectant remains high enough to stop bacterial growth. We call this the Minimum Inhibitory Concentration (MIC). If the bacteria can survive the concentration at a certain distance, they grow right up to that line.
But wait. Some molecules are chunky. Phenolic compounds, common in older disinfectants like Pine-Sol, are relatively large. They struggle to push through the dense forest of agar polymers. Meanwhile, something like hydrogen peroxide is a tiny, nimble molecule. It zips through the agar like a sprinter.
So, a small zone might just mean the disinfectant is a "slow walker," even if it’s incredibly lethal to the bacteria it actually touches. You have to account for molecular weight. If you don't, your disinfectants in zone of inhibition biology experiment results are basically fiction.
✨ Don't miss: Maya How to Mirror: What Most People Get Wrong
Why Bacteria Choice Changes Everything
You can't just pick "bacteria" as a monolith. Most lab kits use Bacillus subtilis or Escherichia coli. There is a world of difference between them.
Gram-positive bacteria (like Bacillus or Staph) have a thick, porous cell wall made of peptidoglycan. They’re usually pretty easy to kill with common disinfectants because the chemicals can soak right in. Gram-negative bacteria (like E. coli or Salmonella) are the "boss fight" of the petri dish. They have an extra outer membrane. It’s a lipid bilayer that acts like a raincoat, flicking away many water-soluble disinfectants.
If you’re testing a "natural" cleaner against E. coli, don't be shocked when the zone is non-existent. It’s not necessarily that the cleaner is "weak." It’s that it can’t pick the lock of the Gram-negative outer membrane.
The Problem with Volatility
Ever tried testing 70% Isopropyl alcohol? It’s a classic choice. But alcohol is a nightmare for this specific experiment. Why? It evaporates.
By the time you’ve finished labeling your plates and popped them in the incubator at 37°C, half of your "active ingredient" might have vanished into the air. This leads to artificially small zones. Pro tip: if you’re using volatile substances, you need to seal the plates quickly, though even then, the results are often wonky compared to non-volatile quaternary ammonium compounds (Quats).
Setting Up the Experiment for Real Accuracy
Forget just "dipping" the disc. That's amateur hour. To get data that actually means something, you need precision.
🔗 Read more: Why the iPhone 7 Red iPhone 7 Special Edition Still Hits Different Today
First, the agar depth must be consistent. Exactly 4mm is the standard for Mueller-Hinton agar (the gold standard for these tests). If your agar is too thin, the disinfectant spreads too far, and your zones look giant. If it’s too thick, the chemical sinks down instead of out, and your zones look tiny.
- Standardize your inoculum. Use a McFarland 0.5 turbidity standard. This ensures you have roughly the same number of "soldiers" (bacteria) on every plate. If one plate has 10 times more bacteria than another, the disinfectant has to work harder, and the zone will shrink.
- The "Dip and Tap" method. When soaking your filter paper discs, don't let them drip. Submerge them, then tap them against the side of the container. A soggy disc will leak fluid across the surface of the agar, ruining the perfect circle and creating a "zone" that’s actually just a puddle.
- Incubation timing. Check your plates at 18–24 hours. If you leave them for three days, some bacteria might start to develop "mutant" colonies inside the zone, or the disinfectant might degrade, allowing a "faint regrowth" that makes measuring impossible.
Interpreting the Data (The Nuance)
We often use a ruler to measure the diameter in millimeters. But what are we actually measuring?
It’s easy to see a clear line. Sometimes, though, you get a "ghost zone." This is an area where the bacteria aren't dead, but they aren't thriving either. They look thin or translucent. In a professional disinfectants in zone of inhibition biology experiment, we only measure the area of complete inhibition. If there’s a light haze, the bacteria are technically "resistant" or "intermediate" to that concentration.
Also, consider the "Lawn." If your bacterial lawn isn't confluent—meaning if there are gaps in the growth—your measurement is garbage. You need a solid, velvety carpet of microbes to see where the "kill line" truly starts.
Common Disinfectants and Their Typical Behavior
- Bleach (Sodium Hypochlorite): Usually creates a very sharp, clear zone. It’s an oxidizer. It tears apart everything—proteins, lipids, DNA. It’s the "sledgehammer."
- Hydrogen Peroxide: Fast-moving, creates large zones, but can be inconsistent if the bacteria produce catalase (an enzyme that breaks down peroxide into water and oxygen). You might literally see tiny bubbles in the agar.
- Essential Oils (Tea Tree, Oregano): These are tricky. They aren't water-soluble. They don't like to move through agar. You’ll often get tiny zones, but that doesn't mean they aren't potent; they're just stuck near the disc.
- Hand Sanitizers: Because of the gel thickeners (like carbomer), the alcohol doesn't diffuse well. Liquid alcohol usually performs "better" in this specific test than gel, even if the alcohol percentage is the same.
Real-World Limitations
Let's be honest: the zone of inhibition test is a "best-case scenario" for the disinfectant. In the real world, bacteria aren't growing on a nice, moist bed of agar. They’re stuck in "biofilms" on a dry countertop or hiding under organic matter like dirt or blood.
Many disinfectants that look like superstars in a petri dish fail in a hospital because they are "inactivated" by organic loads. Bleach is a great example. If there's a lot of "gunk" (protein) on a surface, the bleach spends all its energy reacting with the gunk and has nothing left for the bacteria. The agar plate doesn't show you that.
💡 You might also like: Lateral Area Formula Cylinder: Why You’re Probably Overcomplicating It
Actionable Insights for Your Next Trial
If you want your experiment to be more than just a classroom craft project, change how you approach it.
Instead of just comparing different brands, compare concentrations. Take one disinfectant (like a Quat-based cleaner) and dilute it: 100%, 50%, 25%, 12.5%. Plot the results on a graph. You’ll see a logarithmic relationship between concentration and zone size. That’s real science.
Another move? Test the "Time-to-Kill." The zone of inhibition test shows static inhibition over 24 hours. It doesn't tell you if the disinfectant kills in 30 seconds (which is what you want for a kitchen spray). To test that, you’d need a suspension test, but that's a story for another day.
Your next steps:
- Calibrate your tools: Use a digital caliper instead of a plastic ruler for measurements to the nearest 0.1mm.
- Control the variables: Ensure all your discs are the same diameter (6mm is the standard).
- Reference the charts: Look up the CLSI (Clinical & Laboratory Standards Institute) guidelines. Even though they are for antibiotics, the principles for "Susceptible, Intermediate, and Resistant" categories are the framework for understanding how we quantify microbial death.
- Run a "Negative Control": Always include a disc soaked in sterile water. If you see a zone around the water disc, your agar is contaminated or your water isn't sterile, and your whole experiment is invalid.
Focus on the "why" behind the circle, not just the size. That’s where the real biology happens.