Honestly, sitting in a high school chemistry lab while staring at a mess of letters and numbers feels like trying to solve a Sudoku puzzle where the rules keep changing. You've got your reactants on the left. You've got your products on the right. And for some reason, the atoms just won't line up. It’s frustrating. But learning to balance this chemical equation isn't actually about being a math genius; it’s about accepting that matter is stubborn and refuses to just disappear into thin air.
The Law of Conservation of Mass is the culprit here. Antoine Lavoisier, the French nobleman who basically laid the groundwork for modern chemistry before the French Revolution caught up with him, insisted that you can't create or destroy matter. If you start with four oxygen atoms, you better end with four. It sounds simple. In practice? It’s a headache.
The Mental Block Behind Balancing Equations
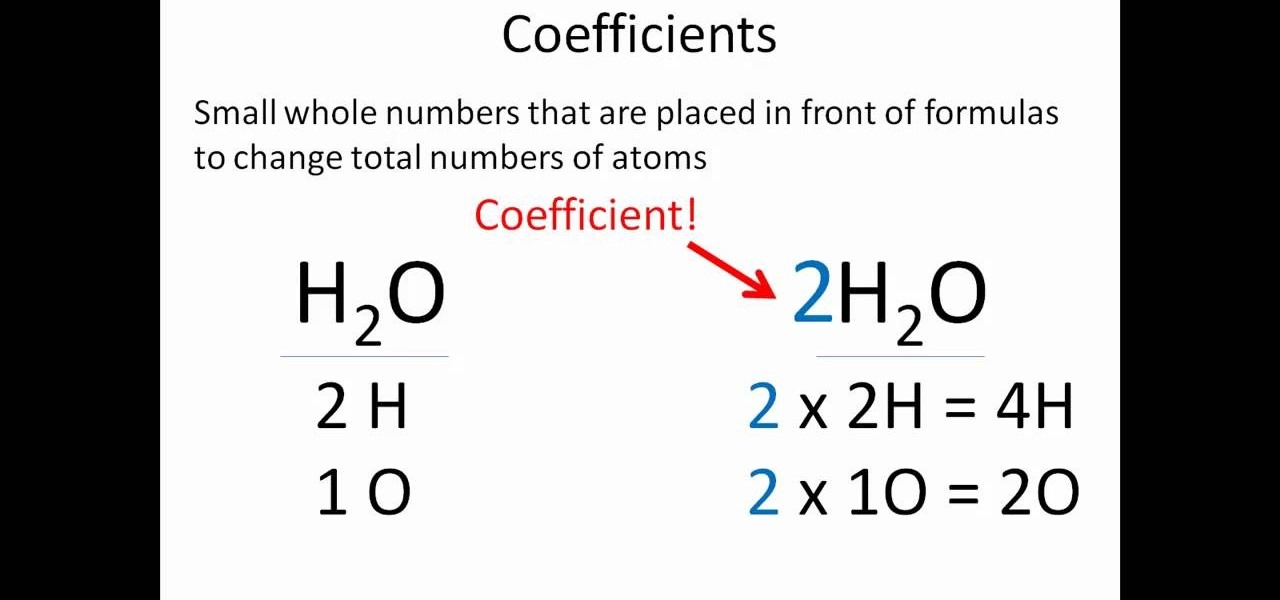
Most people fail at balancing because they try to change the "subscripts." Big mistake. If you change the little $_2$ in $H_2O$, you aren't balancing water anymore—you're making hydrogen peroxide ($H_2O_2$), which is great for cleaning cuts but terrible for staying hydrated. You have to use coefficients. Those are the big numbers out front. They tell you how many molecules you have, not what the molecule is made of.
Think of it like a recipe. If you’re making a sandwich and you have three slices of bread but only two slices of cheese, you don't just shave the bread down to make it fit. You change the quantity of sandwiches. Balancing is just inventory management for the microscopic world.
A Step-by-Step Mess: The Combustion of Propane
Let’s look at a real-world example that everyone uses but few actually "get" on the first try: burning propane. This is the stuff in your backyard grill. The unbalanced version looks like this:
$$C_3H_8 + O_2 \rightarrow CO_2 + H_2O$$
💡 You might also like: How Big is 70 Inches? What Most People Get Wrong Before Buying
If you look at that and feel a slight panic, you’re normal. On the left, we have three carbons. On the right, only one. The hydrogen situation is even worse—eight on the left, two on the right. It’s a disaster.
Start with the heavy hitters
I always tell people to leave Oxygen for last. Oxygen is a "free element" in this equation ($O_2$), which means you can tweak it at the very end without messing up anything else. It's your safety net.
- Fix the Carbon first. We have three on the left, so we put a $3$ in front of the $CO_2$. Now carbons are happy.
- Handle the Hydrogen. We have eight on the left and two on the right ($H_2O$). What times two equals eight? Four. Stick a $4$ in front of the water.
Now, let's look at what we've done to the Oxygen. On the right side, we now have $3 \times 2$ (from the $CO_2$) which is six, plus the $4$ from the water. That’s ten. Since Oxygen comes in pairs on the left, we just need a $5$ in front of the $O_2$.
The result?
$$C_3H_8 + 5O_2 \rightarrow 3CO_2 + 4H_2O$$
It's balanced. It’s clean. It’s satisfying.
📖 Related: Texas Internet Outage: Why Your Connection is Down and When It's Coming Back
Why Does This Even Matter?
You might think this is just busywork for students, but "stoichiometry"—the fancy word for the relationship between reactants and products—runs the world. If an engineer at a pharmaceutical company like Pfizer or Merck messes up the balance of a chemical equation, they could end up with a toxic byproduct instead of a life-saving medicine.
In the world of green energy, balancing equations is how we calculate the efficiency of hydrogen fuel cells. If we want to replace gasoline with hydrogen, we have to know exactly how much energy is released when $2H_2 + O_2$ turns into $2H_2O$. There is no "close enough" in chemical engineering. If the math is off, the engine explodes or the battery dies in ten minutes.
Common Pitfalls and the "Fraction Trick"
Sometimes you get stuck in a loop. You fix one side, and it breaks the other. This happens a lot with combustion reactions where you end up with an odd number of oxygens on one side and an even number on the other.
Don't panic. Use a fraction.
If you need 13 oxygen atoms but they only come in pairs ($O_2$), just use $13/2$ as a temporary coefficient. Then, once the whole equation is "balanced" with that fraction, multiply every single coefficient by two to get rid of the decimal. It’s a shortcut that saves about twenty minutes of trial and error.
👉 See also: Why the Star Trek Flip Phone Still Defines How We Think About Gadgets
The Tools of the Trade
In 2026, we have apps and AI that can balance these in half a second. Photomath and specialized chemistry solvers are everywhere. But relying on them is like using a calculator before you know how to add. You lose the "feel" for the reaction.
When you balance it by hand, you start to see patterns. You notice that polyatomic ions—like Nitrate ($NO_3$) or Sulfate ($SO_4$)—often stay together as a single unit. Instead of counting individual Sulfurs and Oxygens, you just treat the whole "Sulfate" like a single Lego brick. It’s much faster.
Real-World Nuance: It’s Not Always This Simple
In a textbook, equations always balance perfectly. In a real lab, things are messy. You have "limiting reactants." This is the reality where you run out of one ingredient before the others. If you're trying to balance this chemical equation for a real experiment, you're also dealing with "percent yield." You might calculate that you should get 10 grams of product, but because of heat loss or impurities, you only get 8.
Chemistry is a game of theoretical perfection clashing with the chaotic reality of the physical world.
Actionable Steps for Mastering Any Equation
If you want to stop guessing and start balancing like a pro, follow this specific order of operations. It works almost every time.
- List the atoms: Write down exactly how many of each element you have on both sides. Update this list every time you change a coefficient.
- Identify the "Singles": Look for elements that appear in only one molecule on each side. Balance those first.
- Save Oxygen and Hydrogen for the end: They are the most common and usually the most annoying.
- Double-check the math: This sounds obvious, but 90% of errors are simple multiplication mistakes (like thinking $3 \times 3$ is 6).
- Simplify: If your coefficients are 4, 8, and 4, divide them all by 4 to get the lowest whole-number ratio.
The next time you're faced with a daunting string of chemical symbols, remember that it's just a scale. Your job is to make sure the weights match. Once you get the rhythm, it actually becomes kind of addictive. You stop seeing a wall of text and start seeing a puzzle waiting to be solved.
Start with the simplest ones—like the synthesis of water—and work your way up to complex organic combustion. Practice is the only way the logic becomes second nature. Grab a pencil, find a messy equation, and start counting.