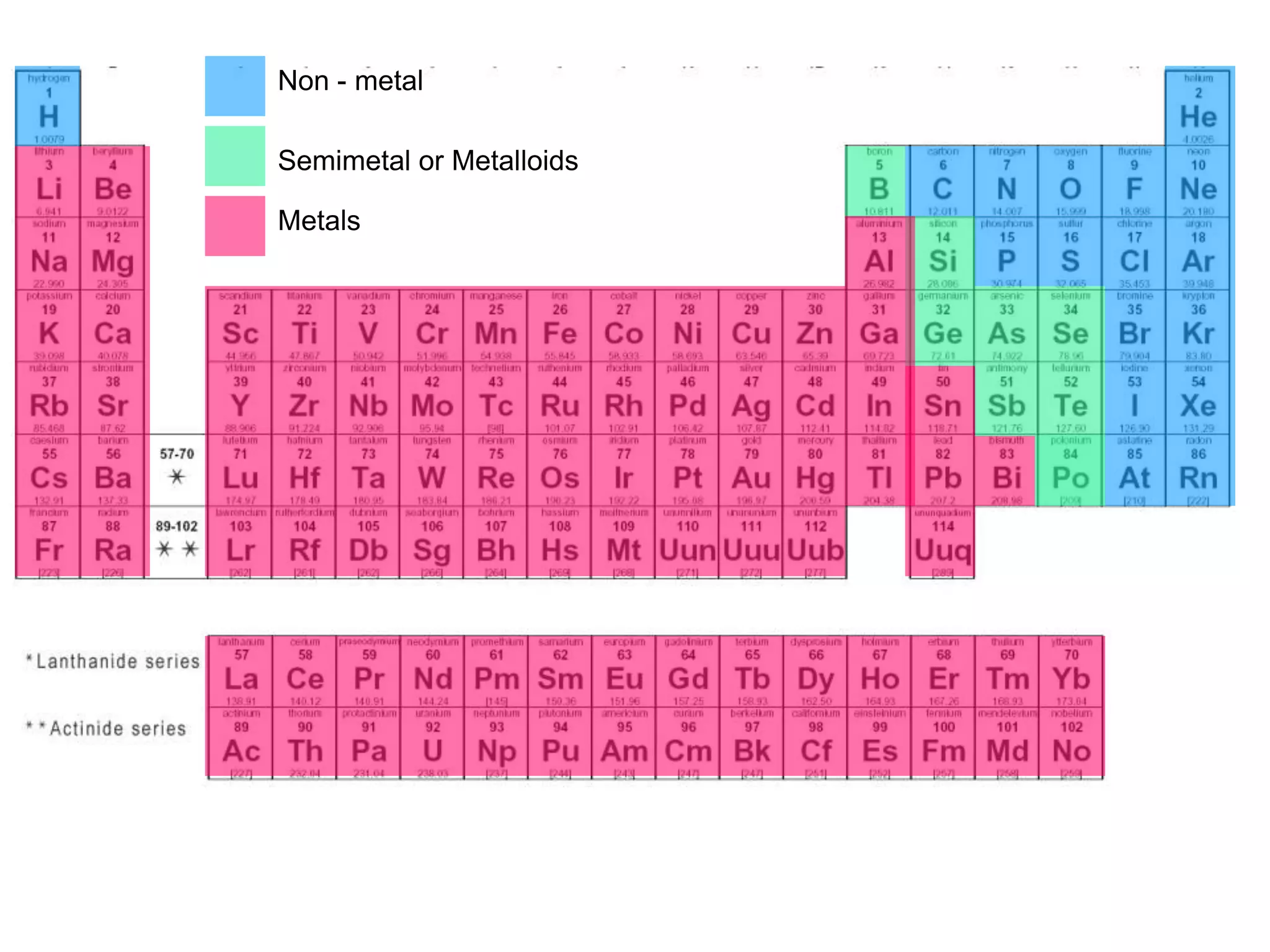
Everything is chemistry. Look at the phone in your hand or the coffee mug on your desk. You’re staring at a giant jigsaw puzzle of elements that shouldn't, by any logical standard, play nice together. But they do. If you've ever looked at a metals and nonmetals periodic table, you’ve seen that jagged, staircase-looking line cutting through the right side. It looks like a mistake. It looks like someone just drew a zig-zag because they ran out of room.
Actually, that line is the most important divider in the known universe.
It separates the stuff that builds skyscrapers from the stuff that keeps us alive. We’re talking about the difference between a chunk of iron and the oxygen filling your lungs. Most people just memorize the colors for a test and forget it. But if you actually understand why these elements sit where they do, you start to see why our technology—and our bodies—actually work.
The Left-Side Heavyweights: Metals
The periodic table is honestly a bit lopsided. About 80% of all known elements are metals. They dominate the landscape. They start all the way at the far left with the Alkali metals—stuff like Lithium and Sodium—and stretch across the vast middle. These are the "socialites" of the atomic world. Why? Because they’re constantly trying to give away their electrons.
Metals are defined by their generosity. In scientific terms, we call this low ionization energy. Basically, they don't hold onto their outer electrons very tightly. This loose grip is why copper wires carry electricity to your charger. The electrons are just drifting in a "sea," flowing wherever the voltage pushes them.
Think about a piece of gold. You can beat it into a leaf so thin that light passes through it. That’s malleability. You can’t do that with a diamond or a piece of sulfur. If you hit sulfur with a hammer, it just turns into a sad yellow powder. Metals are different because their atoms can slide past each other without breaking the overall bond. It’s a weird, fluid-like strength that makes modern infrastructure possible. From the Tungsten in old lightbulbs to the Titanium in a hip replacement, metals are the literal backbone of the physical world.
The Oddballs on the Right: Nonmetals
Once you cross that zig-zag line, things get weird. Nonmetals are the contrarians. They aren't shiny. They don't conduct heat well. Most of them are gases at room temperature. If metals are the builders, nonmetals are the messengers and the fuel.
Take Carbon. It’s the poster child for nonmetals. Depending on how you stack those atoms, you either get the graphite in your pencil or a diamond. Then you have the Halogens, like Fluorine and Chlorine. These guys are the opposite of metals—they are "greedy." They don't want to give away electrons; they want to rip them away from anyone nearby. This high electronegativity makes them incredibly reactive. It’s why Chlorine is so good at killing bacteria in a pool—it literally tears the molecules of the bacteria apart to get those electrons.
Hydrogen is the real troublemaker here. Look at a metals and nonmetals periodic table and you'll see Hydrogen sitting way over on the left, right above the most reactive metals. But it's a gas. It’s a nonmetal. It’s only there because it has one electron, just like Sodium. It’s the ultimate "misfit" of the periodic table, proving that the rules are more like guidelines.
The Staircase: Where Boundaries Blur
The "Metalloids." This is where the magic happens for your MacBook or your smartphone. Elements like Silicon and Germanium sit right on that zig-zag line. They aren't quite metals, and they aren't quite nonmetals. They are semiconductors.
This is huge.
💡 You might also like: All of the Apple Emojis: Why Your iPhone Keyboard Keeps Changing
If Silicon were a perfect metal, your computer would short-circuit instantly because the electricity would just dump through it. If it were a nonmetal, it would be an insulator, and nothing would happen. Because it’s a metalloid, we can "tune" it. We can make it conduct electricity only under certain conditions. That "on-off" capability is the basis of every binary bit of data ever created. Without that specific section of the metals and nonmetals periodic table, we’d still be using vacuum tubes and punch cards.
Honestly, the metalloids are the bridge. They have the luster of metals but the brittleness of nonmetals. They are the chemical "maybe."
Why the Arrangement Actually Matters for SEO and Science
When researchers at places like MIT or the Max Planck Institute look for new materials, they aren't guessing. They use the periodic trends. They know that as you move down a group (a vertical column), the atoms get bigger and the metals get more reactive. This is why Cesium is way more explosive in water than Lithium is.
If you're looking at the metals and nonmetals periodic table to understand battery technology, you’re looking at the top left. Why Lithium? Because it’s small and light. It’s a metal that can move its ions quickly through a liquid. If we used Lead (way further down), the battery becomes a heavy brick. That’s why your car battery is Lead-Acid (heavy, stays in the car) and your phone is Lithium-Ion (light, stays in your pocket).
Common Misconceptions
- "All metals are solid." Nope. Mercury is a liquid at room temperature. Gallium will literally melt in the palm of your hand if you hold it long enough.
- "Nonmetals are weak." Try telling that to a diamond. While most are brittle, the covalent bonds in certain nonmetal structures are the strongest in nature.
- "The line is permanent." Under extreme pressure—like at the core of Jupiter—scientists believe Hydrogen actually turns into a metal. Imagine a metal made of gas. That’s the kind of stuff that keeps physicists up at night.
Chemical Bonding: The Ultimate Marriage
The whole reason we study the metals and nonmetals periodic table is to predict how things will react. When a metal meets a nonmetal, you usually get an ionic bond. The metal gives up an electron, the nonmetal takes it, and they are stuck together by static electricity. That’s table salt (Sodium Chloride).
When two nonmetals meet? They share. That’s a covalent bond. That’s the water you drink and the DNA in your cells. Nonmetals are the masters of complexity because they can share electrons in endless combinations. Metals just tend to form big, repetitive crystals.
How to Use This Knowledge Today
If you're a student, a hobbyist, or just someone curious about how the world is built, stop looking at the table as a chart to memorize. Look at it as a map of "behavioral tendencies."
- Check the Conductivity: If you need to fix a household circuit or understand why your pan has a plastic handle, look at the metal/nonmetal divide. Metals move heat; nonmetals (insulators) block it.
- Predict Reactivity: If you’re cleaning your house, never mix products containing different nonmetals (like bleach and ammonia) without knowing their placement. They "want" electrons so badly they will create toxic gases to get them.
- Material Selection: If you’re 3D printing or building something, the "brittleness" of the nonmetal-heavy side of the table tells you why certain plastics fail where aluminum holds up.
The metals and nonmetals periodic table isn't just a classroom decoration. It’s a cheat code for the physical universe. By knowing where an element sits relative to that staircase, you already know its "personality" before you even see it in a lab.
Next time you see a piece of rusted iron, you aren't just seeing old metal. You're seeing a literal war for electrons where a nonmetal (Oxygen) finally won a fight against a metal (Iron). The table explains the outcome of that battle before it even starts.
Next Steps for Mastery:
- Download a High-Res Periodic Table: Get a version that specifically highlights the "staircase" to visualize the metalloid bridge.
- Explore Periodic Trends: Look into Electronegativity and Atomic Radius to see how the properties of metals and nonmetals change as you move across the rows.
- Observe Your Environment: Identify five items in your room and categorize them as metallic, nonmetallic, or a composite of both to see the table in action.