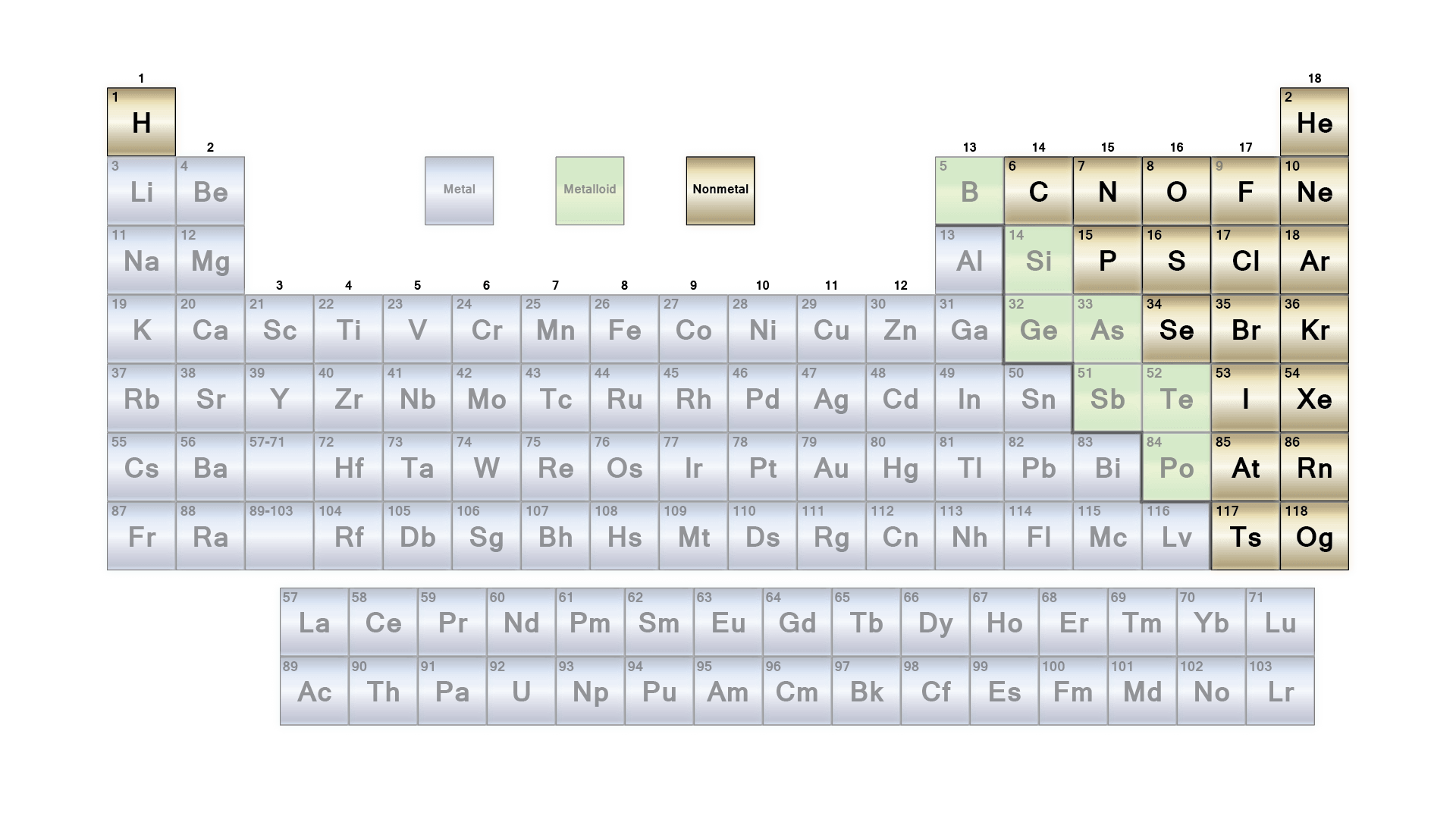
Look at a periodic table. It’s usually color-coded like a preschool map, with a jagged staircase separating the metal and non metal periodic table sections. We're taught in ninth grade that metals are shiny, conductive, and tough, while nonmetals are basically the opposite—gaseous or brittle and insulators. But if you actually dig into the chemistry, that clean line is a total lie. It’s more of a blurred, messy transition zone where elements like Silicon and Germanium play both sides of the fence.
Everything you touch is dictated by this divide. Your phone’s processor exists because silicon refuses to pick a side. Your lungs work because oxygen is a stubborn nonmetal. If we didn't have this specific chemical "divorce" between the two sides of the table, life as we know it would be a metallic soup of nothingness.
The Shiny Side: Why Metals Own the Left
The vast majority of the periodic table—about 80%—is made of metals. They dominate the left and center. Think of them as the extroverts of the atomic world. They’re constantly looking to ditch their outer electrons. This "electron sea" is why you can hammer a piece of gold into a leaf so thin it’s translucent without it shattering.
💡 You might also like: Finding Local Creators: How to Search OnlyFans by Location Without Getting Scammed
You’ve got the Alkali Metals in Group 1, like Lithium and Sodium. They are so eager to lose an electron that they’ll literally explode if they touch water. It’s violent. Then you move into the Transition Metals—the heavy hitters like Iron, Copper, and Gold. These are the elements that built the Bronze Age and the Industrial Revolution. They have high melting points and can carry an electrical charge because those loose electrons move like water through a pipe.
But here is the thing: Mercury is a metal, yet it’s a liquid at room temperature. Gallium will literally melt in the palm of your hand. It’s not all steel girders and hard surfaces. The definition of a "metal" is more about how electrons behave than how the material feels to the touch.
The Quiet Power of Nonmetals
Flip over to the right side of the staircase. This is where things get weird. Nonmetals are the introverts. Instead of giving away electrons, they want to hog them. This "greed" is what creates the strong covalent bonds that hold your DNA together. Carbon is the king here. Depending on how those carbon atoms are stacked, you either get the graphite in your pencil or a diamond that can cut through stone.
Most nonmetals are gases—Hydrogen, Helium, Nitrogen, Oxygen. They don’t conduct heat well. They aren't shiny. If you find a solid nonmetal, like Sulfur, it’s usually crumbly and yellow. It has no "malleability." You hit it with a hammer, and it turns into dust.
Nitrogen makes up 78% of the air you're breathing right now. It's inert, quiet, and essential. But pair it with the right elements, and it becomes the backbone of fertilizers that feed billions. The metal and non metal periodic table distinction isn't just a classification; it's a map of how the universe builds complexity. Metals provide the structure; nonmetals provide the chemistry of life.
The Metalloids: The Fence-Sitters Changing Everything
Right on that jagged staircase sit the Metalloids. Boron, Silicon, Germanium, Arsenic, Antimony, Tellurium. These are the "freaks" of the table. They look like metals but act like nonmetals, or vice versa.
Silicon is the most famous example. It’s shiny and looks metallic, but it’s brittle. Most importantly, it’s a semiconductor. It doesn't conduct electricity as well as Copper, but it doesn't block it like Glass. By "doping" silicon with other elements, we can control exactly when it allows electricity to flow. That’s the "0" and "1" of every computer chip ever made. Without this specific group of elements that refuse to be either metal or nonmetal, you wouldn't be reading this on a screen.
Breaking Down the Real Differences
If we're being honest, the textbooks oversimplify it. Here is how the transition actually works in the real world:
Chemical Behavior
Metals usually form basic oxides. If you dissolve magnesium oxide in water, you get a base. Nonmetals? They form acidic oxides. When sulfur dioxide hits water in the atmosphere, you get acid rain. This is a fundamental rule that helps chemists identify mystery substances without even seeing them.
Lustre and Sonority
Metals are "sonorous." Hit a big chunk of iron with a mallet, and it rings. Hit a chunk of phosphorus, and you just get a dull thud and a mess. Metals also have "lustre"—they reflect light because of those free-roaming electrons on the surface. Nonmetals are generally "dull," though iodine is a weird exception that has a bit of a metallic sheen.
Electronegativity
This is the big one. On the Pauling scale, nonmetals have high electronegativity. They are "electron-hungry." Metals have low electronegativity. Fluorine is the most "greedy" element on the table; it will react with almost anything to get that last electron it needs to be stable.
👉 See also: Google Year in Search: What the Data Actually Says About Us
Common Misconceptions About the Divide
People often think Hydrogen belongs with the metals because it's in Group 1. It’s not. It’s a gas. It’s a nonmetal through and through, but it sits there because it has one lonely electron. On the flip side, some people think all metals are heavy. Lithium is so light it would float on oil.
Another big mistake is assuming the "staircase" is a hard wall. It’s not. Under extreme pressure, things change. Deep inside Jupiter, scientists believe Hydrogen becomes a "metallic" liquid because the pressure is so intense it forces the electrons to behave like they do in a piece of copper. The metal and non metal periodic table labels are really just descriptions of how elements behave under Earth-standard conditions.
Why This Matters for the Future
We are currently in a "Material Science" arms race. We are trying to find new ways to make nonmetals act like metals (like carbon nanotubes) to create ultra-strong, ultra-light materials. We’re also looking at "post-transition metals" to replace toxic lead in electronics.
Understanding this divide helps us predict how elements will bond. If you take a metal from the far left and a nonmetal from the far right (like Sodium and Chlorine), they have such a huge "hunger gap" that they form an ionic bond—resulting in common table salt. If two nonmetals meet, they share electrons, creating the molecules that make up your body.
Moving Beyond the Basics
If you want to actually master the periodic table, stop looking at it as a static chart. It’s a spectrum.
- Get a high-res interactive table. Use tools like Ptable to see how properties like "atomic radius" or "electronegativity" shift as you move from the metallic left to the nonmetallic right.
- Focus on the d-block. The transition metals are where the most complex chemistry happens, especially in industrial catalysis.
- Watch the "Metalloid" zone. If you’re interested in tech or green energy, elements like Tellurium and Antimony are becoming increasingly critical for solar panels and advanced battery tech.
- Experiment with displacement reactions. One of the easiest ways to see the "reactivity series" of metals is to drop a copper wire into a silver nitrate solution. You’ll literally watch the metal "grow" on the wire as the silver ions reclaim their electrons.
The metal and non metal periodic table isn't just for chemistry quizzes. It’s the blueprint for every physical object you’ve ever touched. From the iron in your blood to the silicon in your pocket, the "messy divorce" between these two groups is what makes the universe functional.