Chemistry class usually starts with a lie. Or, if not a lie, a massive oversimplification. Your teacher probably handed you a periodic table and told you everything is balanced. Then they dropped the "strong" label on a bunch of chemicals. You probably assumed "strong" meant "will melt through a floor like the blood in Alien."
Honestly? That’s not what it means at all.
In the world of aqueous solutions, "strong" is just a fancy way of saying a substance is a total quitter. It gives up. When you drop a strong acid into water, it doesn't hold onto its protons. It dissociates completely. Every single molecule breaks apart. Weak acids, on the other hand, are clingy. They keep most of their hydrogen atoms to themselves. This distinction is the backbone of the list of strong acids and strong bases, and if you’re trying to calculate pH or survive a lab practical, getting this list right is non-negotiable.
The Big Seven: Every Strong Acid You Need to Know
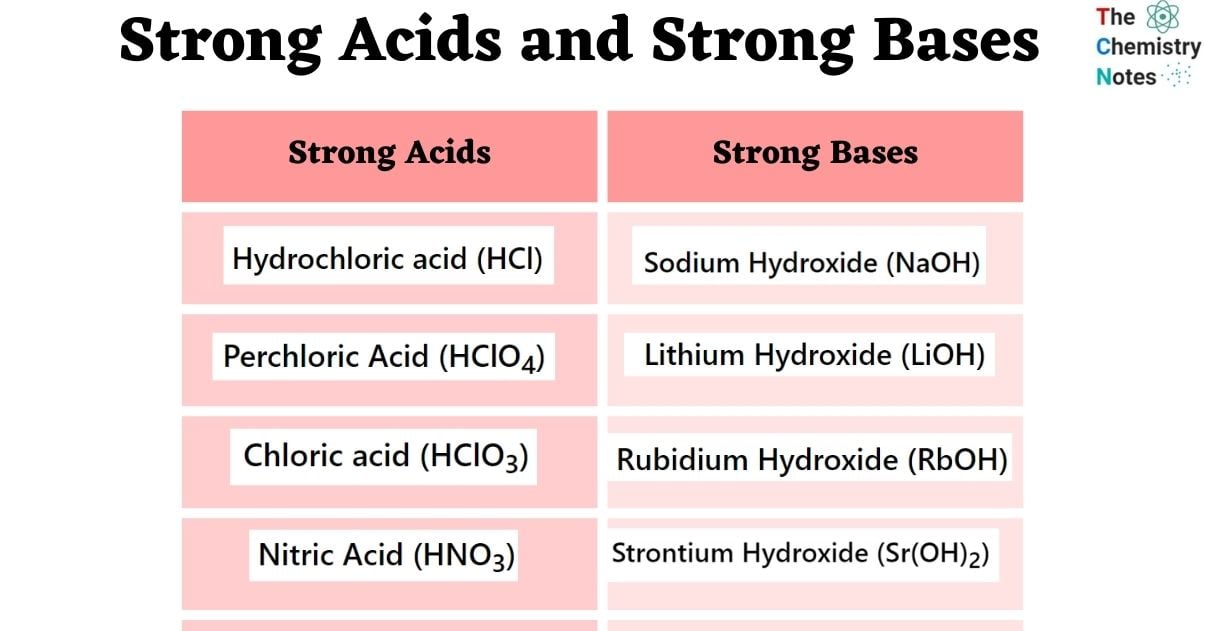
Most people think there are dozens of strong acids. There aren't. There are basically seven. If it isn't on this specific list, you can almost always assume it’s weak.
Let’s look at the "Halogen Trio" first. You've got Hydrochloric acid ($HCl$), Hydrobromic acid ($HBr$), and Hydroiodic acid ($HI$). Notice someone is missing? Fluorine. Even though Fluorine is the most electronegative element on the table, Hydrofluoric acid ($HF$) is actually a weak acid. It’s too stubborn. The bond between Hydrogen and Fluorine is so strong that the molecules don't fully break apart in water. It’ll still eat through glass and ruin your day, but chemically speaking, it's "weak."
Then we get into the oxyacids. This is where things get spicy. You have Perchloric acid ($HClO_4$), Chloric acid ($HClO_3$), Sulfuric acid ($H_2SO_4$), and Nitric acid ($HNO_3$).
👉 See also: Draft House Las Vegas: Why Locals Still Flock to This Old School Sports Bar
Sulfuric acid is a bit of a weirdo. It’s diprotic, meaning it has two hydrogens to give away. But here is the kicker: only the first hydrogen is "strong." Once $H_2SO_4$ drops that first proton to become $HSO_4^-$, it becomes a weak acid. It holds onto that second proton much more tightly. If you're doing high-level titration, forgetting that distinction will wreck your math.
Defining the List of Strong Bases
Strong bases are a little easier to spot if you know your way around the periodic table. They are basically just the hydroxides of the Group 1 and Group 2 metals.
Think of the "S-block" on the left side of your chart. Lithium hydroxide ($LiOH$), Sodium hydroxide ($NaOH$), Potassium hydroxide ($KOH$), Rubidium hydroxide ($RbOH$), and Cesium hydroxide ($CsOH$) are your Group 1 powerhouses. They dissolve easily. They create a massive amount of hydroxide ions ($OH^-$) instantly.
Group 2 is where it gets slightly messy. Calcium hydroxide ($Ca(OH)_2$), Strontium hydroxide ($Sr(OH)_2$), and Barium hydroxide ($Ba(OH)_2$) are considered strong. But if you’ve ever tried to mix Calcium hydroxide in a lab, you know it doesn't dissolve well. It’s "sparingly soluble." However, the small amount that does dissolve dissociates 100%. That is why we keep it on the list.
The Levelling Effect: Why Strength is Relative
Here is something they don't always tell you in the textbooks. In water, all these strong acids look the same.
✨ Don't miss: Dr Dennis Gross C+ Collagen Brighten Firm Vitamin C Serum Explained (Simply)
It’s called the Levelling Effect.
Because water is a base, it reacts with any strong acid to form $H_3O^+$ (hydronium). Since the strong acids all dissociate completely, the strongest "acid" that can actually exist in water is just hydronium. You can't really tell the difference between the "strength" of Hydrochloric acid and Perchloric acid once they are in a dilute aqueous solution. They both just turn into a bucket of hydronium.
To actually see which one is "stronger," you have to move the party to a different solvent, like acetic acid. Only then do you see that Perchloric acid is the real heavyweight champion.
Real World Dangers and Misconceptions
Don't confuse "strong" with "concentrated."
You can have a very dilute solution of Hydrochloric acid that is safer than a super-concentrated solution of Acetic acid (vinegar). "Strong" is a description of chemical behavior, not necessarily immediate lethality. That said, Sodium hydroxide—standard lye—is a strong base and it will dissolve proteins (like your skin) faster than many acids.
🔗 Read more: Double Sided Ribbon Satin: Why the Pro Crafters Always Reach for the Good Stuff
Common household mistakes happen here. People mix cleaners. Don't do that. Bleach is a weak base, but it's reactive. When people start mixing things from the list of strong acids and strong bases with household ammonia or bleach, they create toxic gases like chloramine.
How to Memorize This Without Losing Your Mind
Stop trying to memorize every chemical name in existence. Just learn the outliers.
- Acids: Remember the "Big Seven." If it's not $HCl$, $HBr$, $HI$, $HClO_3$, $HClO_4$, $H_2SO_4$, or $HNO_3$, it's probably weak.
- Bases: Look at the first two columns of the periodic table. If it’s an Alkali or Alkaline Earth metal paired with $OH$, it’s strong (mostly).
- The HF Trap: Never forget that Hydrofluoric acid is the "fake" strong acid. It’s deadly, but it’s chemically weak.
Understanding this list isn't just about passing a test; it’s about predicting how the world reacts. Whether you're balancing a pool, neutralising a spill, or just curious why your stomach doesn't dissolve itself (shout out to the mucous lining protecting you from $HCl$), these chemicals are the literal foundation of our physical reality.
Next Steps for Mastery
Check your cleaning supplies at home. Look for ingredients like Sodium Hydroxide or Sulfuric acid (often in drain cleaners). Read the safety data sheets (SDS) online for those specific products to see how their "strength" dictates the personal protective equipment required. If you're a student, practice drawing the dissociation equations for the Group 2 bases, paying close attention to the stoichiometry of the $OH^-$ ions, as this is the most common place where calculation errors occur in acid-base chemistry.