Energy doesn't just pop into existence because we want it to. It doesn't vanish when we're done with it, either. That’s the core of the first law of thermodynamics, and honestly, it’s the most humbling realization in all of physics. You’ve probably heard it phrased as "energy cannot be created or destroyed, only transformed." It sounds simple. It sounds like something you memorize for a tenth-grade quiz and then promptly forget while trying to calculate the trajectory of a falling apple. But if you actually sit with it, the implications are staggering.
Everything—from the smartphone in your pocket to the supernova exploding in a distant galaxy—is just a massive accounting project. The universe is a closed system with a fixed budget. Every time you turn on a light, you aren't "making" light. You're just trading one form of energy for another.
The Math Behind the Magic
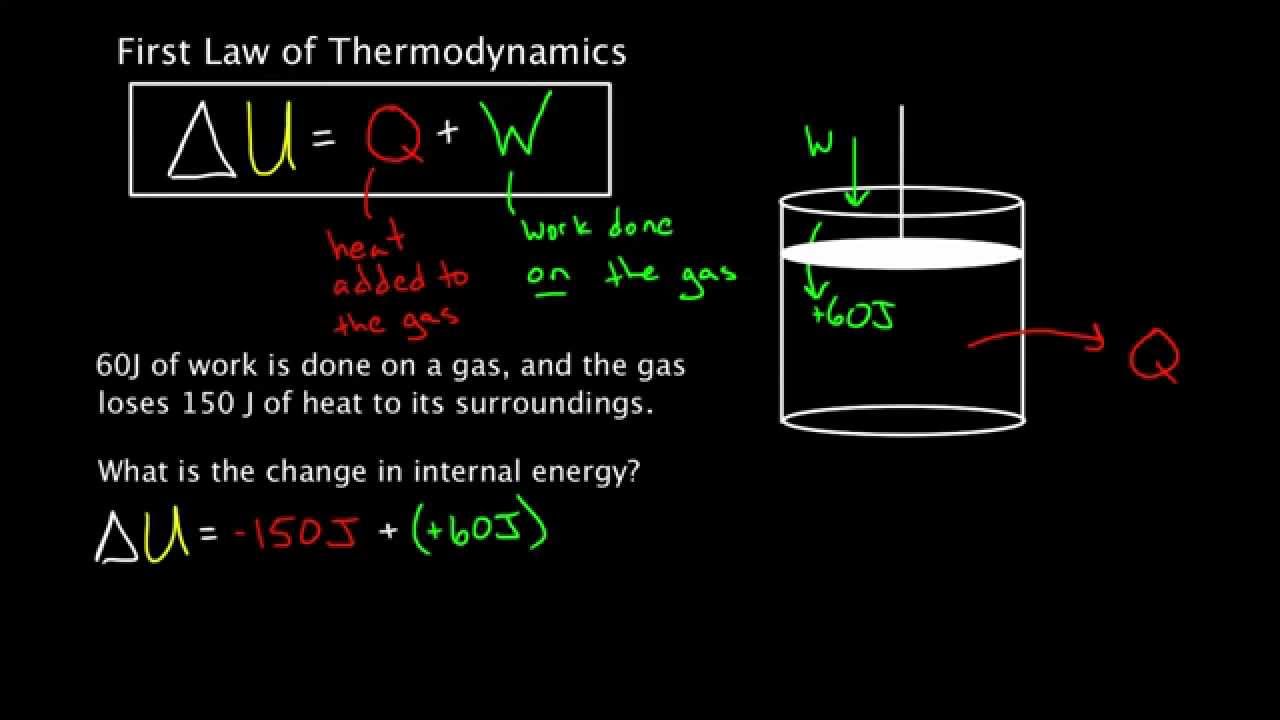
If we want to get technical, and we should, the first law of thermodynamics is essentially a statement about the conservation of energy. It’s usually expressed with a deceptively short equation:
$$\Delta U = Q - W$$
In this scenario, $\Delta U$ represents the change in the internal energy of a system. $Q$ is the heat added to the system, and $W$ is the work done by the system. It’s a balance sheet. If you put heat into a steam engine, that energy has to go somewhere. It either heats up the water molecules (increasing internal energy) or it pushes a piston (doing work).
It never just disappears into the void.
James Prescott Joule, a brewer by trade and a scientist by obsession, was the guy who really nailed this down in the 1840s. Before him, people thought heat was a fluid called "caloric." They thought it flowed from hot things to cold things like water. Joule realized that mechanical work—like stirring a vat of water—could actually raise the temperature. He proved that heat and work are just two different ways of moving energy around. This was revolutionary. It bridged the gap between the physical movement we see and the microscopic vibrations we feel as warmth.
Why You Can't Build a Perpetual Motion Machine
People have been trying to cheat this law for centuries. You’ve seen the YouTube videos: "Infinite Energy Generator!" or "Self-Running Magnet Motor!"
👉 See also: Frontier Mail Powered by Yahoo: Why Your Login Just Changed
They are all lies.
The first law of thermodynamics is the ultimate buzzkill for inventors looking for a free lunch. To get work out of a machine, you must put energy in. Even if you built a perfectly frictionless wheel, it could only keep spinning with the energy it already has. It couldn't power your house while it spins because that would be "creating" energy out of thin air. It’s why every "over-unity" device ever "invented" eventually stops or is revealed as a scam involving hidden batteries. Nature doesn't offer credit.
Real-World Chaos: From Car Engines to Your Lunch
Think about your car. You pump gas into it. That gasoline is a concentrated store of chemical potential energy. When the spark plug ignites the vapor, a chemical reaction happens. The energy stored in those molecular bonds is released as heat. That heat expands gases, which push the pistons.
But here’s the kicker: not all that energy goes into moving the car forward.
A huge chunk of it—sometimes up to 70%—is "lost" as heat through the tailpipe or the radiator. This doesn't mean the first law is broken. It just means the energy shifted into a form that isn't useful for driving to the grocery store. It’s still in the universe. It’s just warming up the air around the highway instead of turning the axles.
Your body does the exact same thing.
You eat a sandwich. That’s your input ($Q$). Your body breaks down the carbohydrates and fats. Some of that energy keeps your heart beating and your brain firing ($\Delta U$). Some of it allows you to walk across the room ($W$). And a lot of it is radiated off your skin as heat. If you’ve ever been in a crowded gym, you’ve felt the first law of thermodynamics in action. Everyone is converting chemical energy from breakfast into heat and kinetic energy, and the room gets sweltering as a result.
✨ Don't miss: Why Did Google Call My S25 Ultra an S22? The Real Reason Your New Phone Looks Old Online
The Nuance of "Internal Energy"
We talk about internal energy like it's one thing, but it’s actually a chaotic mess of microscopic activity. It’s the kinetic energy of atoms bouncing around. It’s the potential energy of the bonds holding molecules together. When we say a system has "internal energy," we're really talking about the sum of all those tiny, invisible movements.
Rudolf Clausius, another heavyweight in the thermodynamics world, was instrumental in refining this. He realized that it wasn't enough to just talk about heat and work; you had to account for the state of the matter itself. This led to the realization that energy isn't just "stuff" you move around; it's a property of the system's state.
Misconceptions That Stick Around
One of the biggest mistakes people make is confusing "energy" with "power."
Energy is the total amount of "work-ability" you have. Power is just how fast you use it. You can have a tiny battery with a lot of energy that releases it slowly, or a massive explosion that releases the same amount of energy in a millisecond. The first law doesn't care about the speed. It only cares about the totals.
Another weird one? The idea that cold is a "thing."
Physically speaking, there is no such thing as "cold." There is only the absence of heat. When you open a window in the winter, "cold" isn't coming in. Heat is leaving. The energy is transferring from the high-energy environment (your warm living room) to the low-energy environment (the freezing street). The first law tracks that migration.
Thermodynamics in the Digital Age
You might think 19th-century steam engine laws don't apply to your MacBook Pro.
🔗 Read more: Brain Machine Interface: What Most People Get Wrong About Merging With Computers
Wrong.
Data centers are the modern battlefield of the first law of thermodynamics. Every calculation a computer performs requires a tiny bit of electrical energy. Most of that energy eventually turns into heat. This is why massive server farms for Google or OpenAI are built near rivers or in cold climates—they need a way to move that "waste" heat away so the chips don't melt. We are literally limited in how fast our AI can think by how effectively we can manage the energy balance dictated by thermodynamics.
Why Should You Care?
It’s easy to view this as purely academic, but it’s the most practical framework for understanding life. It teaches us about efficiency. Since we know energy can't be created, we realize that the goal of technology isn't to "make" energy, but to lose as little as possible during the transfer.
- Home insulation: It’s just a way to slow down the transfer of heat energy.
- Electric vehicles: They are more "efficient" because they lose less energy to heat than internal combustion engines do.
- Metabolism: Understanding how your body converts fuel can change how you approach fitness and diet.
Actionable Insights for Moving Forward
Understanding the conservation of energy isn't just for physicists; it's a mental model for efficiency in the real world.
Audit your energy leaks. Whether it's your home's HVAC system or your own personal schedule, look at where "input" isn't resulting in "useful output." In a house, this means using a thermal camera to find where heat is escaping. In your life, it means recognizing when you're "stirring the water" (doing work) without actually "raising the temperature" (achieving a goal).
Stop looking for "free" solutions. In business and physics, if something looks like it's producing value out of nowhere, you’re likely missing a hidden cost. The first law teaches us that there is always a trade-off. If a new technology claims to be 100% efficient, it’s violating the laws of physics. Always ask: "Where is the energy coming from, and where is it going when it's done?"
Study the Second Law next. The first law says you can't win; the second law says you can't even break even. While the first law tells us energy is conserved, the second law explains why it becomes less "useful" over time (entropy). To truly understand why the universe works the way it does, you have to see how these two laws dance together.
Invest in heat management. As we move toward more powerful computing and electric transport, the bottleneck is rarely "getting the energy." It’s "getting rid of the heat." Learning the basics of heat transfer—conduction, convection, and radiation—is a superpower in any technical field.