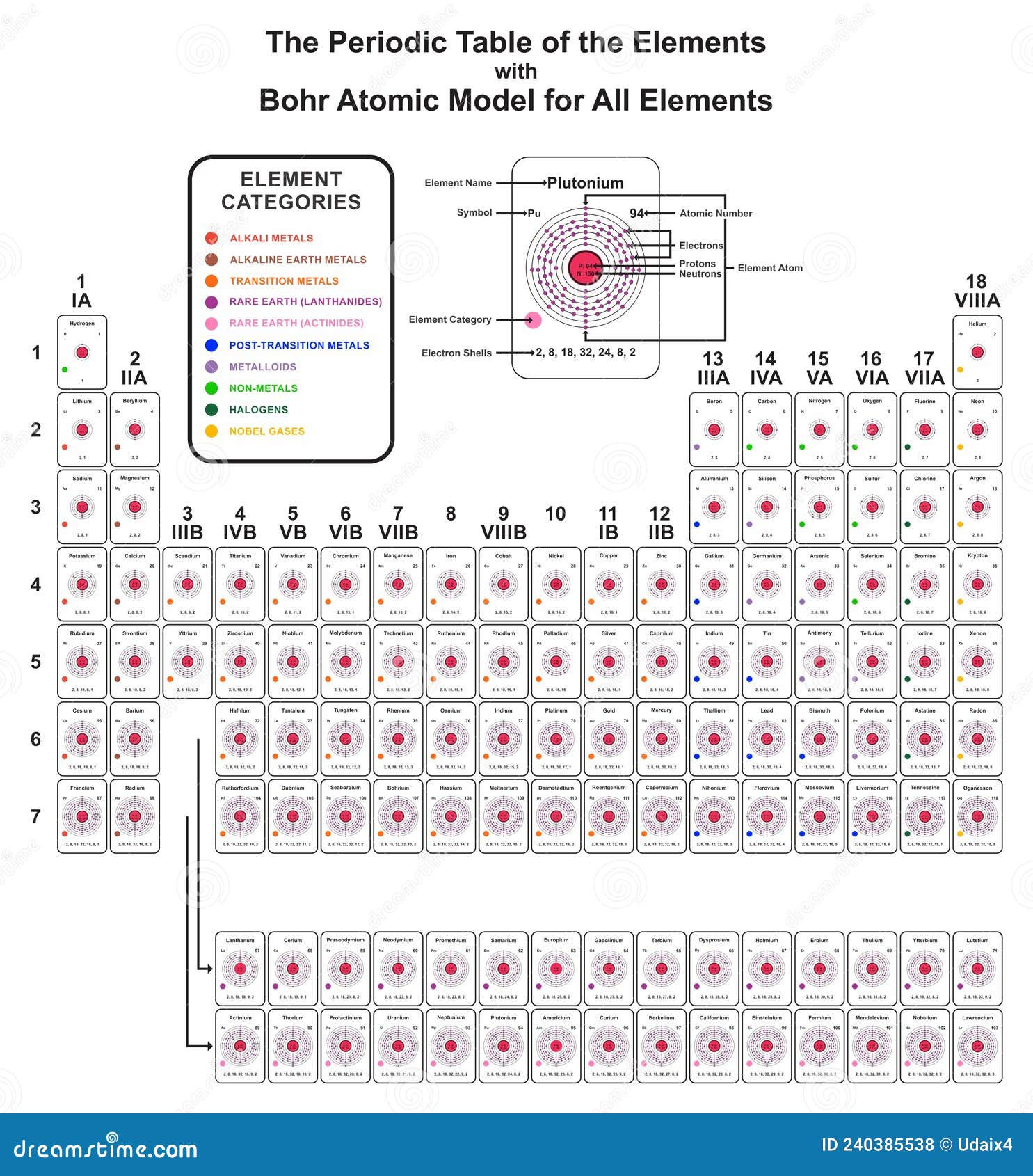
Niels Bohr was a bit of a rebel. In 1913, when he first sat down to sketch out what an atom actually looks like, he wasn't just guessing; he was trying to fix a massive problem in physics. Most people think of the Bohr atomic model periodic table as a relic of a bygone era. You might have seen it in a dusty textbook—those neat little rings around a nucleus that look like a solar system. It’s pretty, sure. But it’s also the foundation for how we organize every single element we know today.
Without Bohr, the periodic table is just a weirdly shaped grid of letters. With Bohr, it’s a roadmap.
How Bohr Fixed the "Exploding Atom" Problem
Before Bohr, scientists were in a bit of a panic. Ernest Rutherford had already proven that atoms had a dense center, but according to the laws of physics at the time, electrons should have just spiraled into the nucleus and caused the whole thing to blow up. Atoms shouldn't have existed.
Bohr stepped in and basically said, "What if they can't?"
He proposed that electrons occupy specific orbits. Think of them like steps on a ladder. You can stand on the first step or the second step, but you can’t hover in the space between them. This was huge. When we talk about the Bohr atomic model periodic table, we are talking about the moment chemistry met quantum mechanics. This "quantization" meant that electrons have specific energy levels.
Wait. Why does that matter for the periodic table?
It matters because those energy levels (or shells) are exactly why the table is shaped the way it is. The first shell can only hold two electrons. Look at the first row of your periodic table. Hydrogen. Helium. Just two. That’s not a coincidence. It's the physical limit of the first Bohr orbit.
The Shell Game of Chemical Reactivity
If you’ve ever wondered why Sodium (Na) is so reactive that it explodes in water, while Neon (Ne) just sits there doing absolutely nothing, you have the Bohr model to thank for the explanation.
Bohr’s genius was realizing that an atom's personality—its "chemical behavior"—is dictated by its outermost shell. These are the valence electrons. Atoms are basically obsessed with being stable. To an atom, stability means having a full outer shell.
Sodium has one lonely electron in its outer shell. It desperately wants to get rid of it. On the flip side, Chlorine is missing just one. When they meet, it’s a match made in heaven (or a lab), creating table salt. This concept of shell-filling is the literal engine that drives the rows and columns of the periodic table.
Rows represent the number of shells.
Columns represent the number of electrons in that outer shell.
Why the Model Isn't "Wrong," Just Incomplete
If you talk to a high-level quantum physicist, they might scoff at Bohr. They’ll tell you electrons aren't little planets; they are "probability clouds." They’ll bring up the Schrödinger equation and Heisenberg’s Uncertainty Principle.
And they aren't wrong.
$$H\psi = E\psi$$
✨ Don't miss: The Marvin Rebirth: Why This Python Legend is Suddenly Back from the Dead
The math above (the Schrödinger equation) provides a much more accurate picture of the electron's position. But here’s the thing: it’s nearly impossible to visualize. For 99% of us, the Bohr atomic model periodic table provides a functional mental map that works for predicting reactions and understanding basic bonding.
Bohr knew his model was a simplified version. He wasn't trying to map the universe; he was trying to explain why Hydrogen emits specific colors of light when you shock it with electricity. He realized that when an electron jumps from a high-energy "step" to a lower one, it spits out a photon of light. The color of that light is a direct result of the distance between those steps.
Real World Impact: From LEDs to Lasers
This isn't just academic fluff. The way we design LED lights and lasers is based on this "jump" between Bohr's orbits.
- We push energy into an atom (excitation).
- The electron jumps to a higher Bohr shell.
- It falls back down almost instantly.
- It releases energy as a specific wavelength of light.
If we didn't understand the quantized nature of these shells, we wouldn't be able to tune lasers for surgery or create the screens you are staring at right now. The periodic table tells us which elements are best at this. Gallium, for instance, is a superstar in the semiconductor world because of its Bohr-predicted electron configuration.
Misconceptions That Get Taught in School
Honestly, most schools skip the best parts. They make you memorize that the shells go 2, 8, 18, 32. But they rarely explain why.
They also often fail to mention that the Bohr model starts to fall apart once you get past Calcium. Once you hit the transition metals—those elements in the middle of the table like Iron and Gold—things get messy. Electrons start filling sub-shells in a way that Bohr's simple rings can't quite handle.
This is where the "Aufbau Principle" comes in. It’s a fancy way of saying electrons fill the lowest energy levels first, but sometimes those levels overlap. Even so, the Bohr model is the "gateway drug" to this more complex reality. You have to understand the rings before you can understand the clouds.
Is the Bohr Model Still Relevant in 2026?
Absolutely. Even as we move toward more advanced computational chemistry, the Bohr atomic model periodic table remains the primary teaching tool worldwide. Why? Because it’s intuitive. It allows a student to look at Carbon (Atomic Number 6) and immediately see:
- 2 electrons in the first shell.
- 4 electrons in the second shell.
- Needs 4 more to be "happy."
That simplicity is powerful. It allows us to predict how Carbon will bond with Oxygen to make $CO_2$. It turns a wall of data into a logical system.
Actionable Insights for Students and Educators
If you are trying to master chemistry or just want to understand the universe a bit better, don't just memorize the table. Build it.
- Map the Valence: Next time you look at the periodic table, ignore the names. Look at the column number. That tells you the "Bohr Story" of that element’s outer shell.
- Visualize the Jump: When you see a "Neon" sign, realize you are literally seeing Bohr's orbits in action. The gas inside is having its electrons kicked up and down shells.
- Acknowledge the Limit: Use Bohr to understand the why of the first 20 elements. After that, be ready to embrace the "cloud" (orbitals) for the more complex metals.
The Bohr model was never meant to be the final word on the atom. It was a bridge. It bridged the gap between the old physics of things we can touch and the new physics of things we can only calculate. It turned the periodic table from a list into a logic puzzle.
To really "get" chemistry, you have to appreciate the elegance of these orbits. They may not be perfectly accurate descriptions of physical space, but they are perfect descriptions of how energy works in our world. Start by sketching out the first three rows of the table using Bohr circles. You'll see patterns emerge that you never noticed before—patterns that define the very fabric of the material world.
---