You probably remember that drawing from middle school. It’s the one where an atom looks like a tiny solar system. There is a dense nucleus in the middle, and electrons are zooming around it in perfect little circles. It’s iconic. But here is the thing: it’s actually wrong. Mostly.
Despite its flaws, the bohr atomic model definition remains the bedrock of how we teach chemistry and physics today. Why? Because Niels Bohr, back in 1913, did something truly radical. He took a messy, collapsing concept of the atom and forced it to follow the rules of the brand-new world of quantum mechanics. He basically saved the atom from disappearing.
What is the Bohr Atomic Model Definition, Really?
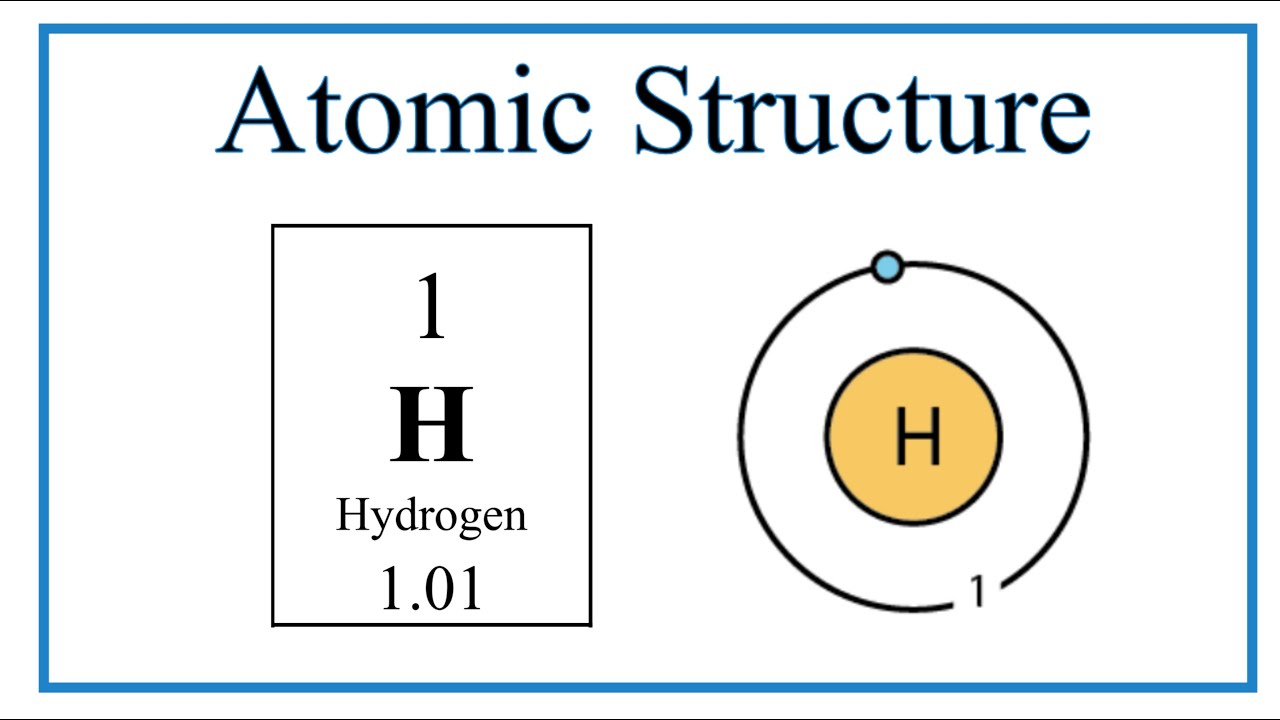
In its simplest form, the Bohr model describes an atom as a small, positively charged nucleus surrounded by electrons that travel in circular orbits. Think of it like planets orbiting the sun, but with a weird twist. In space, a planet can technically orbit at any distance. In Bohr’s world, electrons are stuck.
Bohr proposed that electrons can only exist in very specific "stationary states" or energy levels. They can’t just hang out in the middle. They are either on Level 1, Level 2, or Level 3. It’s like standing on the rungs of a ladder; you can be on the first rung or the second, but if you try to stand in the space between them, you’re going to fall.
This was a massive leap. Before this, Ernest Rutherford had shown the atom had a nucleus, but his model had a huge problem. According to classical physics, an electron circling a nucleus should constantly lose energy. It should spiral inward and smash into the nucleus in a fraction of a second. Everything in the universe should have collapsed. Bohr just decided, "No, it doesn't do that." He postulated that as long as an electron stays in its orbit, it doesn't radiate energy.
The Quantum Leap: It's Not Just a Metaphor
The coolest part of the bohr atomic model definition is how it explains light. If you’ve ever seen a neon sign or watched fireworks, you’re seeing Bohr’s theory in action.
When an atom gets hit with energy—like electricity or heat—an electron gets "excited." It jumps from a low-energy orbit to a higher one. But it can’t stay there forever. It’s unstable. When it eventually drops back down to its original spot, it has to get rid of that extra energy. It spits it out as a photon of light.
The color of that light depends entirely on how far the electron fell. A big drop might create violet light. A small hop might be red. Because each element has its own specific "ladder" of energy levels, each element creates a unique "fingerprint" of colors. This is why hydrogen always glows with the same specific lines when you look at it through a prism. It’s consistent. It’s predictable. It’s honestly kind of beautiful.
The Math Behind the Orbits
Bohr didn't just guess. He calculated the angular momentum of these electrons. He suggested that the angular momentum ($L$) is quantized, meaning it only comes in whole-number multiples of a specific value.
$$L = n \frac{h}{2\pi}$$
In this equation, $n$ is the principal quantum number (1, 2, 3...), and $h$ is Planck's constant. This was the first time someone successfully mashed up classical mechanics with quantum theory. It worked perfectly for hydrogen. It was a masterpiece of "good enough" science that paved the way for everything that came after.
Where Bohr Got It Wrong (And Why Scientists Don't Care)
If you talk to a modern quantum chemist, they’ll tell you electrons don't move in circles. They aren't little billiard balls. Instead, electrons are more like "clouds" of probability. This is the Heisenberg Uncertainty Principle at work. You can't actually know where an electron is and where it’s going at the same time.
Bohr’s model also fails the moment things get complicated. It works for hydrogen because hydrogen only has one electron. But try to use the bohr atomic model definition to predict the behavior of Oxygen or Iron? It falls apart. The math doesn't hold up once you have multiple electrons pushing and pulling on each other.
[Image comparing the Bohr model with the modern quantum mechanical electron cloud model]
He also couldn't explain the "Zeeman Effect"—where spectral lines split into several components when a magnetic field is present. Or why some lines are brighter than others. To fix these, we eventually needed the Schrödinger equation and the full complexity of quantum wave mechanics.
✨ Don't miss: Whose Number Is This? How to Track Down That Mystery Caller Without Getting Scammed
So, why do we still learn it? Because it’s the most intuitive way to understand valence shells and chemical bonding. When you see a Lewis dot structure or talk about "filled shells" in chemistry, you are using Bohr’s logic. It’s a mental map that is 90% accurate for 100% of basic chemistry.
The Human Side of the Discovery
Niels Bohr wasn't working in a vacuum. He was part of an incredible era of discovery at the University of Manchester and later in Copenhagen. He was taking the "Plum Pudding" model of J.J. Thomson and the "Saturnian" models of others and refining them.
Interestingly, Bohr was known for being incredibly slow and deliberate. He would rewrite his papers dozens of times. He was obsessed with getting the language right because he knew he was describing things that defied common sense. He famously said that if you aren't shocked by quantum theory, you haven't understood it.
Practical Insights and How to Use This Knowledge
Understanding the bohr atomic model definition isn't just for passing a chemistry quiz. It’s the foundation for modern technology.
- Spectroscopy: This is how we know what stars are made of. By looking at the light from a star billions of miles away, we can see the "Bohr jumps" happening in its atoms and identify the elements present.
- Lasers: The very word "Laser" (Light Amplification by Stimulated Emission of Radiation) is essentially an application of Bohr’s theory of electron transitions.
- Chemistry Basics: If you want to understand why atoms bond, you have to understand the energy levels Bohr described. Noble gases are "happy" because their Bohr shells are full.
To truly grasp this, stop thinking of the orbits as paths. Think of them as energy states.
If you're a student or an enthusiast, the next step is to look at a periodic table. Notice the rows. Those rows actually correspond to the "n" levels in Bohr’s model. Row 1 has two elements because that first Bohr orbit can only hold two electrons. Row 2 has eight because the next level holds eight.
Actionable Next Steps
- Visualize the Jump: Next time you see a "neon" sign (which might actually be argon or helium), realize you are literally seeing electrons falling down a ladder.
- Compare Models: Look up the "Schrödinger atom" right after reading this. Seeing the transition from Bohr’s "rings" to Schrödinger’s "clouds" is the best way to understand how science evolves.
- Check the Spectrum: Use a cheap diffraction grating or even the back of a CD to look at a fluorescent light bulb. You’ll see the distinct lines of color that Bohr’s model first explained.
The Bohr model is a bridge. It’s not the final destination of physics, but you can’t get to the advanced stuff without crossing it first. It turned the chaos of the subatomic world into an organized, tiered system that we could finally begin to manipulate.
Final Thought: We often discard old theories when new ones arrive, but the Bohr model is different. It’s a "working fiction." It’s a simplified map of a very complex territory, and for most of us, that map is exactly what we need to navigate the world of matter.