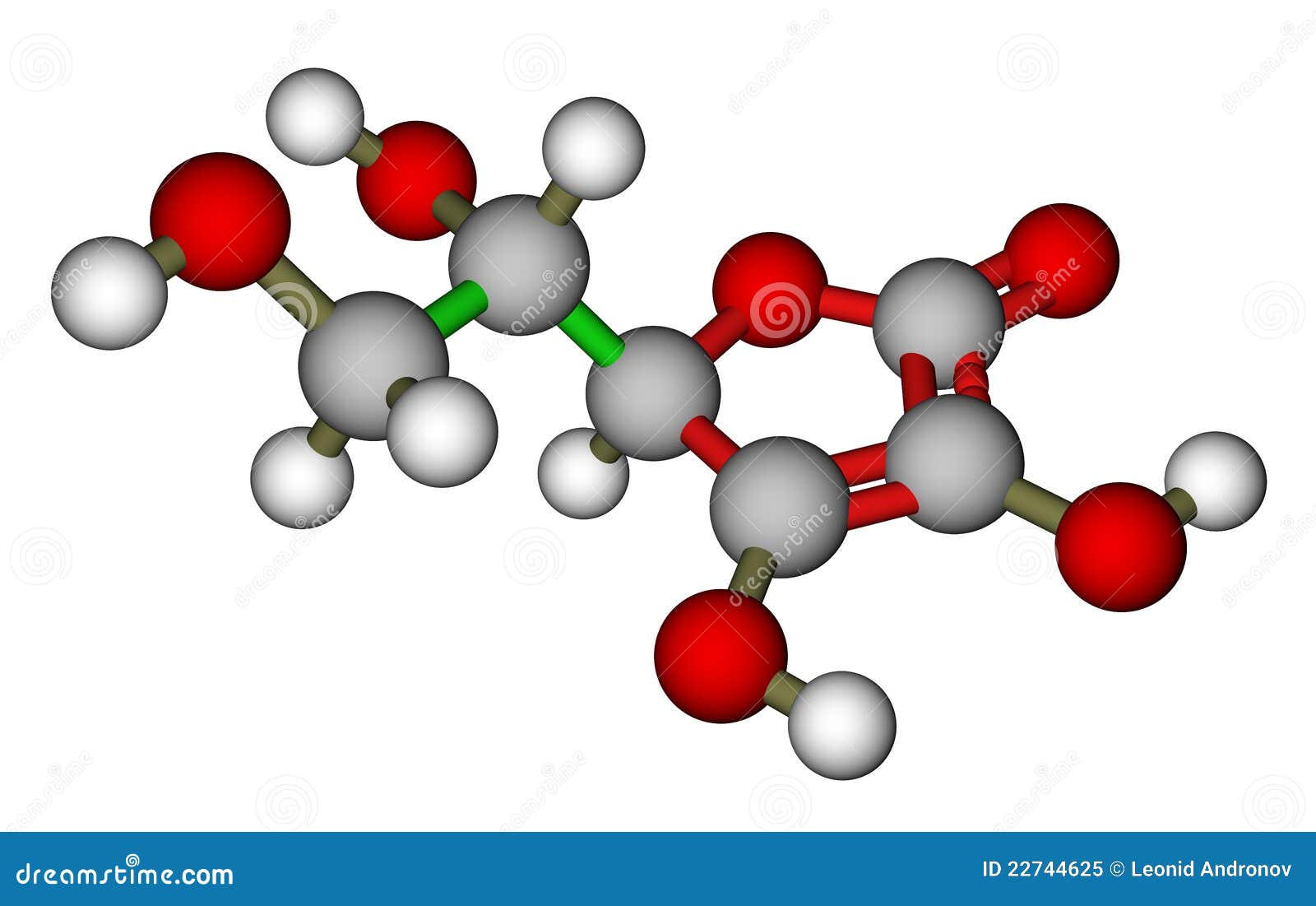
You probably know it as Vitamin C. Most people just grab a bottle of orange-flavored gummies and call it a day. But if you’re actually looking at the science—maybe you're a student, a curious biohacker, or working in a lab—the numbers start to matter. Specifically, we're talking about the ascorbic acid molecular weight. It's exactly 176.12 g/mol.
Does that number sound random? It isn't.
That specific mass dictates how the molecule behaves in your body, how it dissolves in water, and how much you actually need to see a physiological change. It’s the difference between a supplement that works and one that just gives you expensive urine.
What the Ascorbic Acid Molecular Weight Tells Us About Chemistry
At its heart, ascorbic acid is a simple six-carbon compound. Its chemical formula is $C_6H_8O_6$. When you add up the atomic weights of six carbons, eight hydrogens, and six oxygens, you land right on that 176.12 mark.
Chemistry is weirdly precise. If you change even one atom, the "weight" shifts, and the molecule stops being Vitamin C. It becomes something else entirely. This weight is essential for stoichiometry. If a researcher is trying to figure out how many molecules of Vitamin C are needed to neutralize a certain amount of free radicals in a petri dish, they can't just "eyeball" it. They use the molecular weight to convert grams into moles.
Actually, let's talk about the structure for a second. It's a hexose derivative. It looks a bit like a sugar molecule, which is why some animals can actually synthesize it from glucose. Humans? We lost that ability somewhere along the evolutionary line. We’re one of the few mammals that have to eat our Vitamin C because we lack the enzyme L-gulonolactone oxidase. It's a bummer, honestly.
Why 176.12 g/mol is a "Sweet Spot"
A molecular weight of 176.12 is relatively small. In the world of pharmacology, smaller is often better for absorption. Because ascorbic acid isn't a massive, clunky protein, it can move through cellular membranes with the help of specific transporters like SVCT1 and SVCT2.
If the weight were significantly higher, the body would struggle to shuttle it into the brain or the adrenal glands, where it's needed most. It’s light enough to be water-soluble, which is why you can stir it into your morning juice, but that also means your kidneys are constantly trying to get rid of it. You don't store it like Vitamin D or A. It's a "use it or lose it" situation.
The Physical Reality of Pure Ascorbic Acid
When you see "ascorbic acid" on a label, you’re looking at a white to pale yellow crystalline powder. It’s slightly acidic—obviously—with a pH of about 2 to 3 if you dissolve it at a 5% concentration.
Scientists like Linus Pauling, who was famously obsessed with Vitamin C, spent decades arguing about dosages. Pauling believed in "megadosing," taking grams upon grams of the stuff. Now, while the medical community has largely walked back from the idea that 10,000mg a day cures the common cold, the molecular weight remains the foundational metric for all these studies.
- Density: Usually around 1.65 g/cm³.
- Melting Point: It starts to decompose at about 190°C (374°F).
- Solubility: About 33g will dissolve in 100mL of water.
If you’re trying to formulate a skin serum or a shelf-stable juice, these physical constants are your bible. You can't ignore the math. If you've ever had a Vitamin C serum turn brown, that's oxidation. The $C_6H_8O_6$ is losing electrons and turning into dehydroascorbic acid. The weight stays nearly the same, but the function is gone.
Does the "Weight" Change with Different Forms?
This is where people get confused. You’ll see "Sodium Ascorbate" or "Calcium Ascorbate" on supplement bottles. These are "buffered" versions.
They aren't pure ascorbic acid.
Sodium ascorbate, for example, has a molecular weight of 198.11 g/mol. Why? Because you’ve swapped a hydrogen atom for a sodium atom. This makes it less acidic on the stomach, but it also means that a 1,000mg tablet of sodium ascorbate actually contains less "active" Vitamin C than a 1,000mg tablet of pure ascorbic acid. You're paying for the weight of the sodium, too.
Beyond the Lab: Why Should You Care?
You might think this is just for people in white coats. Not really.
If you are a gardener, you might use ascorbic acid to neutralize chlorine in water before watering sensitive plants. If you're a baker, you use it as a dough conditioner to strengthen the gluten. In both cases, knowing the ascorbic acid molecular weight helps you calculate the exact concentration needed to trigger the chemical reaction.
👉 See also: Why Asin Sushi Parasites Inside the Body X Ray Photos Keep Going Viral
Too much? You waste money. Too little? The reaction doesn't happen.
In the human body, the "weight" also dictates the osmotic effect. If you take a massive dose of Vitamin C—say, 5,000mg at once—the concentration of those 176.12 g/mol molecules in your gut becomes too high. Water rushes into your intestines to dilute it. The result? "Bowel tolerance" issues. Basically, you get diarrhea.
Real-World Bioavailability
A study published in the American Journal of Clinical Nutrition by Levine et al. showed that once you go above 200mg in a single dose, the percentage of absorption actually starts to drop. The transporters in your gut get "saturated." They can only carry so many of those 176.12-weight molecules at a time.
It’s like a subway station during rush hour. It doesn't matter how many people are waiting on the platform; only a certain number fit on the train.
Surprising Details About Molecular Stability
One thing most people miss is that the molecular weight is fixed, but the stability is incredibly fragile. Ascorbic acid is a "reducing agent." It loves giving away electrons.
When you cook vegetables, you aren't changing the molecular weight of the Vitamin C, but you are destroying the molecule itself through heat and oxidation. Boiling broccoli can leach up to 50% of the Vitamin C into the water. Because the ascorbic acid molecular weight makes it so water-soluble, it literally just swims away from your food and ends up down the drain.
- Steam your vegetables instead of boiling.
- Keep your Vitamin C supplements in a dark, cool place.
- If your serum smells like "metallic hot dog water" (a common complaint with Skinceuticals-style formulas), that's often the sign of the chemistry shifting.
The Verdict on the Numbers
The number 176.12 is the DNA of Vitamin C's behavior. It determines how much fits in a capsule, how fast it dissolves in your stomach, and how effectively it mops up oxidative stress in your cells.
If you're looking to apply this knowledge, start by checking your supplements. Are you taking pure ascorbic acid, or a heavier buffered salt? If you’re using it for skincare, is the concentration (based on molecular mass) high enough to actually penetrate the skin barrier—usually between 10% and 20%?
Next Steps for Accuracy:
- Calculate your actual intake: If you use a buffered form like Calcium Ascorbate, remember that only about 89% of that weight is actual Vitamin C. Adjust your dose accordingly if you're targeting a specific therapeutic level.
- Check your serum pH: For those 176.12 g/mol molecules to actually get into your skin, the solution needs a pH below 3.5. If it's higher, the molecule stays ionized and won't cross the lipid barrier.
- Watch the color: At the first sign of a yellow or orange tint in your liquid Vitamin C products, the molecular structure has failed. Toss it. It’s no longer the ascorbic acid you paid for.