Look at your phone. Seriously, just stare at it for a second. You’re holding a tiny, high-tech rock made of metal and non metal elements that were forged in the hearts of dying stars billions of years ago. It’s wild when you think about it that way. Most people remember the Periodic Table as that colorful, intimidating chart hanging in their high school chemistry room, usually smelling faintly of floor wax and teenage angst. We memorized the symbols, took the test, and promptly forgot everything.
But here’s the thing: understanding the divide between these two groups isn't just for lab coats. It’s the reason your car doesn't melt when you drive it and why your lungs don't fail when you breathe.
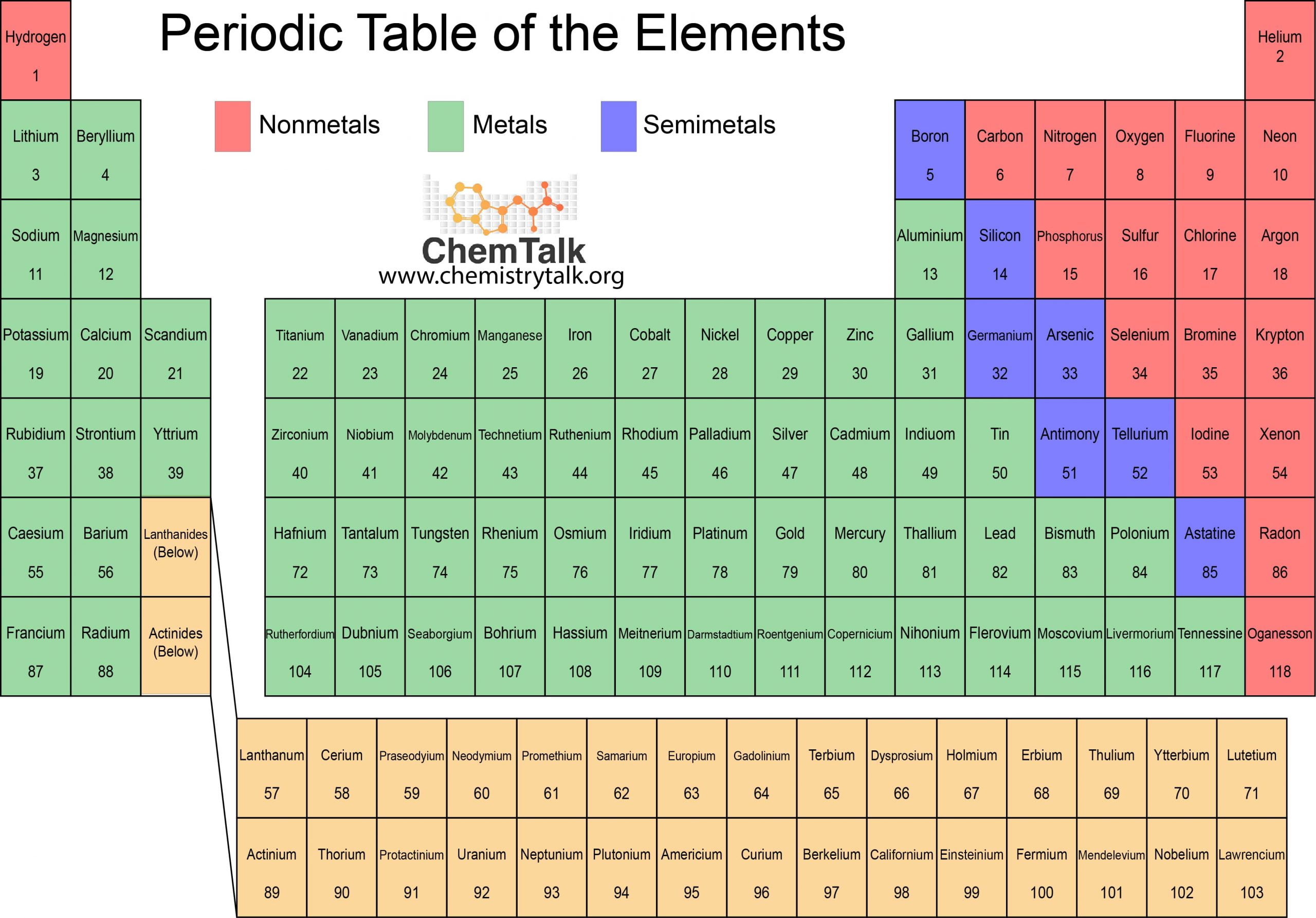
The Great Divide: What’s the Real Difference?
Basically, the universe is sorted by how atoms handle their electrons. Metals are the "generous" ones. They have these loose outer electrons that just kinda wander around, which is why they conduct electricity so well. Non metals? They’re the hoarders. They want to grab electrons and keep them.
You’ve got the metals on the left and center—shiny, tough, and dependable. Then you’ve got the non metals tucked away on the right, plus Hydrogen, which is basically the weirdo of the family that sits by itself.
Metals like Gold (Au) or Iron (Fe) are usually solid at room temperature. Mercury is the oddball exception, staying liquid because its electrons are moving so fast they don't want to bond properly. Non metals, on the other hand, are a mixed bag. You've got gases like Oxygen (O), solids like Sulfur (S), and one lonely liquid, Bromine (Br).
Why the "Luster" Matters
Ever noticed how a silver spoon gleams but a piece of charcoal (Carbon) just looks... flat? That’s luster. Metals reflect light because those free-roaming electrons I mentioned earlier act like a mirror. Non metals are mostly "dull." They absorb light or let it pass through. Honestly, if everything were a metal, the world would be blindingly bright. If everything were a non metal, we’d be living in a very brittle, dark place.
The Secret Strength of Non Metal Elements
We tend to celebrate metals because they’re big and structural. We talk about the "Iron Age" or "Bronze Age." But non metals are the actual architects of life.
Carbon is the undisputed king here.
💡 You might also like: That Gritty Picture of a Robber: Why Most Security Footage Looks So Bad
Without Carbon, there’s no DNA. No proteins. No you. It has this incredible ability to form four bonds at once, making it the perfect Lego brick for building complex molecules. When you arrange Carbon atoms one way, you get the graphite in your pencil. Rearrange them into a crystal lattice under intense pressure, and you get a diamond. It’s the same element, just a different outfit.
Then there’s Nitrogen (N). It makes up 78% of the air you’re breathing right now. It’s remarkably stable, which is good, because if it were as reactive as Oxygen, the atmosphere would basically be one giant explosion waiting to happen.
- Oxygen: The ultimate "taker." It’s highly electronegative, meaning it loves stealing electrons from metals. That’s why your bike rusts. It’s just Oxygen bullying the Iron.
- Phosphorus: Essential for ATP, the battery pack for your cells.
- The Halogens: Elements like Chlorine. Incredibly reactive. They’re the "bad boys" of the non metal world, always looking for a fight (or a bond).
Metals: The Backbone of Everything Heavy
If non metals are the software of life, metals are the hardware.
Copper (Cu) is the unsung hero of the modern world. Every time you flip a light switch, you’re relying on the "sea of electrons" inside copper wiring. Because the atoms in a metal are arranged in a regular, repeating pattern but the electrons are free to flow, electricity moves through them like water through a pipe.
Transition Metals: The Heavy Hitters
The middle of the Periodic Table is where the "Transition Metals" live. Elements like Titanium (Ti), Tungsten (W), and Platinum (Pt). These are the elements with high melting points and serious density.
Tungsten is a freak of nature. Its melting point is about 3,422°C ($6,192°F$). That’s why it was used in lightbulb filaments for a century; it could get white-hot without turning into a puddle.
Where the Magic Happens: Metalloids
There’s a zigzag line on the table that separates these two groups. The elements sitting right on that line are called Metalloids. They’re the "maybe" elements.
Silicon (Si) is the celebrity of this group.
Silicon is a semiconductor. It doesn't conduct electricity as well as Copper, but it doesn't block it like Sulfur. By "doping" Silicon with other elements, engineers can control exactly when it allows electricity to pass through. This is the "on/off" switch for every computer chip ever made. Without this specific group of elements that blur the line between metal and non metal, you wouldn't be reading this on a screen. You’d be reading it on a piece of paper (which is mostly Carbon, Hydrogen, and Oxygen—all non metals).
The Misconception of "Pure" Elements
People often think elements exist in nature as pure chunks. "I found a piece of Iron!" Well, probably not.
Most metals are so eager to give away their electrons that they’ve already bonded with non metals in the crust. You find Iron Oxide (Ore), not pure Iron bars. Refining metal is basically the process of "persuading" the metal to take its electrons back from the non metals it’s bonded with. It takes massive amounts of energy—usually heat—to break those bonds.
🔗 Read more: Microsoft Surface Pro 12 inch: Why the Smallest Pro is Still the Most Disruptive
- Smelting: Using Carbon (Coke) to rip the Oxygen away from Iron ore.
- Electrolysis: Using raw electricity to force Aluminum to separate from its oxide.
It’s a constant tug-of-war.
The Weird Physics of Alloys
We rarely use pure metals in real life because they’re actually kind of "meh" on their own. Pure Gold is too soft to wear. Pure Iron is relatively brittle and rusts instantly.
We make Alloys.
An alloy is a mixture of a metal with other elements (often non metals). Steel is the big one. It’s mostly Iron, but it has a tiny amount of Carbon—a non metal—mixed in. That Carbon sits in the gaps between the Iron atoms, acting like a structural glue that prevents the Iron layers from sliding past each other. This makes the metal much harder and stronger.
Stainless steel goes a step further by adding Chromium. The Chromium reacts with Oxygen to form a microscopic, invisible layer of "rust" that actually protects the rest of the metal from corroding further. It’s a self-healing shield.
Environmental Impact and Scarcity
We’re running into a bit of a problem. Technology is getting hungrier for specific metals and non metals that aren't exactly easy to find.
Lithium (Li) is a metal, but it’s so light it can float on water. It’s essential for batteries. Rare Earth Elements like Neodymium are what make the magnets in electric car motors work.
On the non metal side, we’re actually facing a Helium shortage. Helium is a Noble Gas. It doesn't bond with anything. Once it’s released into the atmosphere, it’s so light that it literally floats off into space. We’re losing it forever every time we fill a party balloon. That’s a problem because liquid Helium is the only thing cold enough to keep MRI machines and particle accelerators running.
Actionable Insights: How to Use This Knowledge
Knowing the difference between these elements isn't just trivia; it has practical applications for how you handle materials in your daily life.
- Cooking: Understand why you use stainless steel (hard, resists acid) vs. copper (fast heat transfer). Never put acidic foods (like tomatoes) in a reactive metal pan like unlined Aluminum, or the non metal Oxygen in the food will pull the metal atoms into your dinner.
- Maintenance: Use "sacrificial anodes" (like Zinc) on boats or water heaters. Zinc is a metal that is "more generous" with its electrons than Iron. The Oxygen will attack the Zinc first, leaving your important structures alone.
- Gardening: Plants need a specific balance of non metals (Nitrogen, Phosphorus, Potassium). If your leaves are yellowing, it’s likely a Nitrogen deficiency—the plant can’t build the "hardware" it needs to process sunlight.
- Safety: Never mix cleaning chemicals like Bleach (contains Chlorine) with Ammonia. These non metals react violently to form toxic gases that can damage your lungs in seconds.
The Next Steps for You
Next time you’re out, try to spot the "interplay." Look at a bridge and see the Steel (Metal + Non metal). Look at your garden and think about the Nitrogen cycle.
If you want to go deeper, check out the work of Theodore Gray. His book The Elements is a visual masterpiece that shows these substances in their raw form. Also, the Royal Society of Chemistry has an interactive Periodic Table that explains the specific uses of the more obscure transition metals like Rhenium or Tantalum.
✨ Don't miss: Why Weather Radar Abilene TX Often Misses the Full Story
Understanding these materials is like seeing the code of the matrix. Once you see the divide between the givers (metals) and the takers (non metals), the physical world starts to make a lot more sense.