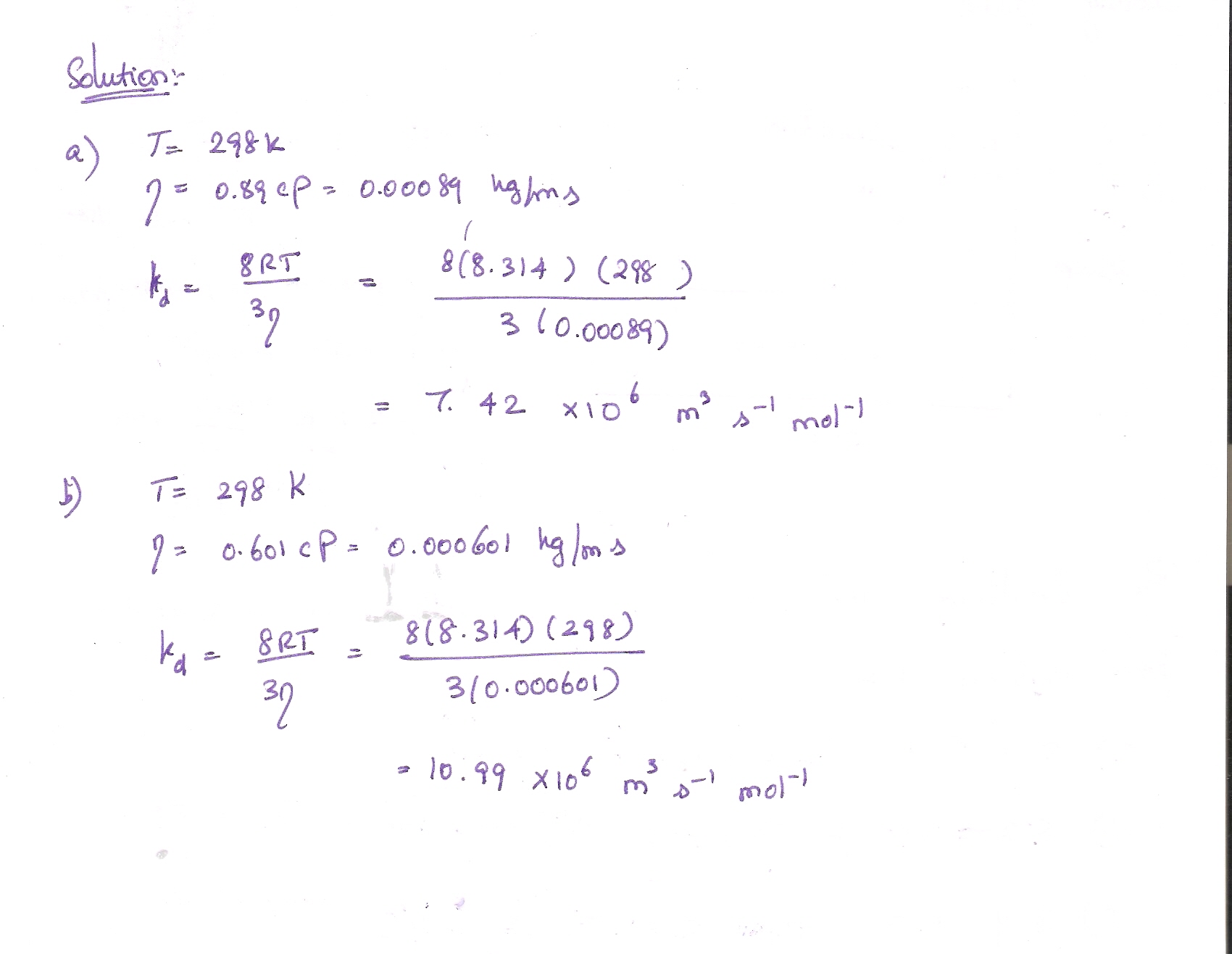You’re standing in a kitchen. Someone opens a bottle of peppermint oil across the room, and suddenly, you smell it. It’s not instant, but it’s fast. That’s diffusion. It's the messy, chaotic, yet predictable movement of particles from where they are crowded to where they have some breathing room. If you’re a student, a lab tech, or just a nerd trying to figure out why your perfume doesn't reach the back of the car fast enough, you need to know how to calculate rate of diffusion.
It isn't just about speed. It’s about mass.
Thomas Graham, a Scottish chemist back in the 1800s, figured out the "why" behind this. He realized that lighter gases are basically the sprinters of the molecular world, while the heavy stuff drags along like a tired toddler. If you want to get technical—and we have to if we want the right answer—you're looking at a relationship between the rate and the square root of the molar mass.
The Basic Math of Graham’s Law
Most people trip up because they think the relationship is linear. It’s not. If a gas is four times heavier than another, it doesn't move four times slower. It moves two times slower. Why? Because the formula uses a square root.
The standard formula looks like this:
$$\frac{Rate_1}{Rate_2} = \sqrt{\frac{M_2}{M_1}}$$
In this equation, $Rate_1$ and $Rate_2$ are the diffusion rates of two different gases. $M_1$ and $M_2$ are their respective molar masses. Notice the flip. The mass of gas 2 goes on top of the mass of gas 1 inside that radical. If you swap those, your entire calculation goes into the trash. Honestly, that’s the most common mistake in undergrad chemistry labs.
Let’s say you’re comparing Hydrogen ($H_2$) and Oxygen ($O_2$). Hydrogen is light. Oxygen is heavy. Hydrogen has a molar mass of about 2 g/mol. Oxygen is sitting around 32 g/mol.
✨ Don't miss: New DeWalt 20V Tools: What Most People Get Wrong
When you plug those in:
$$\frac{Rate_H}{Rate_O} = \sqrt{\frac{32}{2}} = \sqrt{16} = 4$$
This tells you that Hydrogen diffuses four times faster than Oxygen. It's clean. It's elegant. It also explains why a helium balloon goes flat way faster than one filled with regular air. Helium atoms are tiny and fast; they find the microscopic holes in the latex and bail out before the nitrogen and oxygen even know what’s happening.
What Actually Is a "Rate" Anyway?
In science, "rate" usually means something per unit of time. When we talk about how to calculate rate of diffusion, we are usually looking at the amount of gas passing through a certain area or the distance a gas travels over time.
If you're in a lab setting, you might measure the distance ($d$) a gas travels in a tube. In that case, the rate is $d/t$ (distance divided by time). If the time is the same for both gases, the formula simplifies even further because the time variables cancel out. You're just comparing distances.
Fick’s Law: When Things Get Complicated
Graham's Law is great for gases in a vacuum or simple setups. But the real world is sticky. If you’re looking at how nutrients move through a cell membrane or how pollutants spread in a lake, you need Adolf Fick’s work.
Fick’s First Law of Diffusion is the big one for liquids and biological systems. It states that the flux ($J$)—which is just a fancy word for the amount of stuff moving through a surface—is proportional to the concentration gradient.
The formula is:
$$J = -D \frac{d\phi}{dx}$$
🔗 Read more: Memphis Doppler Weather Radar: Why Your App is Lying to You During Severe Storms
- J is the diffusion flux.
- D is the diffusion coefficient.
- dphi/dx is the concentration gradient.
That negative sign isn't a typo. It’s there because diffusion happens from high concentration to low concentration. It’s moving "downhill."
The diffusion coefficient ($D$) is the wildcard here. It changes depending on the temperature, the viscosity of the fluid, and the size of the particles. If you heat up a cup of water, the $D$ value for sugar goes up because the molecules are vibrating like they’re at a rave. More energy equals faster movement.
The Temperature Factor Everyone Forgets
You can't talk about how to calculate rate of diffusion without mentioning Kelvin. Not Celsius. Never Celsius.
Kinetic Molecular Theory tells us that the average kinetic energy of a gas is directly proportional to its temperature. If you crank the heat, you're pumping energy into those molecules. They move faster.
However, even at the same temperature, different molecules move at different speeds. This is the root mean square speed ($v_{rms}$).
$$v_{rms} = \sqrt{\frac{3RT}{M}}$$
- R is the ideal gas constant.
- T is temperature in Kelvin.
- M is molar mass in kilograms per mole (watch your units!).
If you double the temperature (in Kelvin), the speed doesn't double. It increases by a factor of $\sqrt{2}$, or about 1.41. It’s a diminishing return, sort of.
Real World Messiness: Effusion vs. Diffusion
Technically, there’s a difference between diffusion and effusion, though people use the terms interchangeably in casual conversation.
💡 You might also like: LG UltraGear OLED 27GX700A: The 480Hz Speed King That Actually Makes Sense
Effusion is a gas escaping through a tiny hole into a vacuum. Think of a tire leak.
Diffusion is gases mixing together. Think of someone smoking a cigarette in the next room.
The math for Graham's Law works for both, but it's "cleaner" for effusion. In true diffusion, molecules are constantly bumping into each other. These collisions slow everything down. A molecule might be moving at hundreds of meters per second, but it only travels a few nanometers before it slams into another molecule and gets knocked off course. This is called the "mean free path."
If you’re trying to calculate the rate in a crowded room, you have to account for the "tortuosity" or the obstacles in the way. In soil science, for example, gas diffuses slower because it has to wind through tiny pores between dirt particles.
Practical Steps for Accurate Calculations
If you're sitting down to actually run these numbers, don't just wing it.
- Check your units. Molar mass should usually be in g/mol for Graham's Law ratios, but if you're using the $v_{rms}$ formula, you often need kg/mol to match the Joules in the gas constant.
- Identify your "Knowns." Are you given the time it took for a gas to travel? Or the mass of the gas? If you have the formula of the gas (like $CO_2$), you already have the mass. Just look at a periodic table. Carbon is 12, Oxygen is 16. $12 + (16 \times 2) = 44$ g/mol.
- Set up the ratio correctly. Put the rate you want to find on top. If you want to know how much faster Gas A is than Gas B, Gas A’s rate goes on top. That means Gas B’s mass goes on top on the other side of the equals sign.
- Solve for the unknown. Usually, this involves squaring both sides if the unknown is under the radical, or simple cross-multiplication.
Why This Matters Outside the Lab
It’s easy to think this is just academic fluff. It isn't.
In the medical field, the rate of oxygen diffusing into your blood across the alveolar-capillary membrane is literally the difference between life and death. If that membrane thickens due to disease (like pulmonary fibrosis), the "distance" ($x$ in Fick’s law) increases. As the distance increases, the rate of diffusion drops. Patients feel short of breath because the oxygen can't make the jump fast enough.
In the tech world, semiconductor manufacturing relies on the controlled diffusion of impurities (doping) into silicon wafers. If engineers couldn't calculate the exact rate at which phosphorus or boron diffuses into silicon at 1000 degrees Celsius, your smartphone wouldn't exist. They use these exact formulas to determine how long to bake the chips.
Even in the food industry, when you marinate a steak, you're waiting on diffusion. Salt moves faster than larger flavor molecules. This is why a short marinade only salts the surface but doesn't actually "flavor" the center of the meat.
Actionable Insights for Your Next Calculation
- Use the Inverse Square Root Rule: Always remember that if you want to double the rate of diffusion by changing the gas, you need a gas that is four times lighter.
- Convert to Kelvin Immediately: If your problem gives you 25°C, write down 298.15K before you do anything else. Using Celsius will break the entire equation.
- Molar Mass is King: Keep a periodic table handy. You cannot calculate these rates without knowing exactly what molecules you are dealing with.
- Mind the Medium: If you're calculating diffusion in a liquid, Graham's Law won't be enough. You'll need to look up the specific diffusion coefficient ($D$) for that substance in that specific liquid at that specific temperature.
The math might feel heavy, but the concept is simple: things move from where it’s crowded to where it’s not, and the lighter they are, the faster they go.