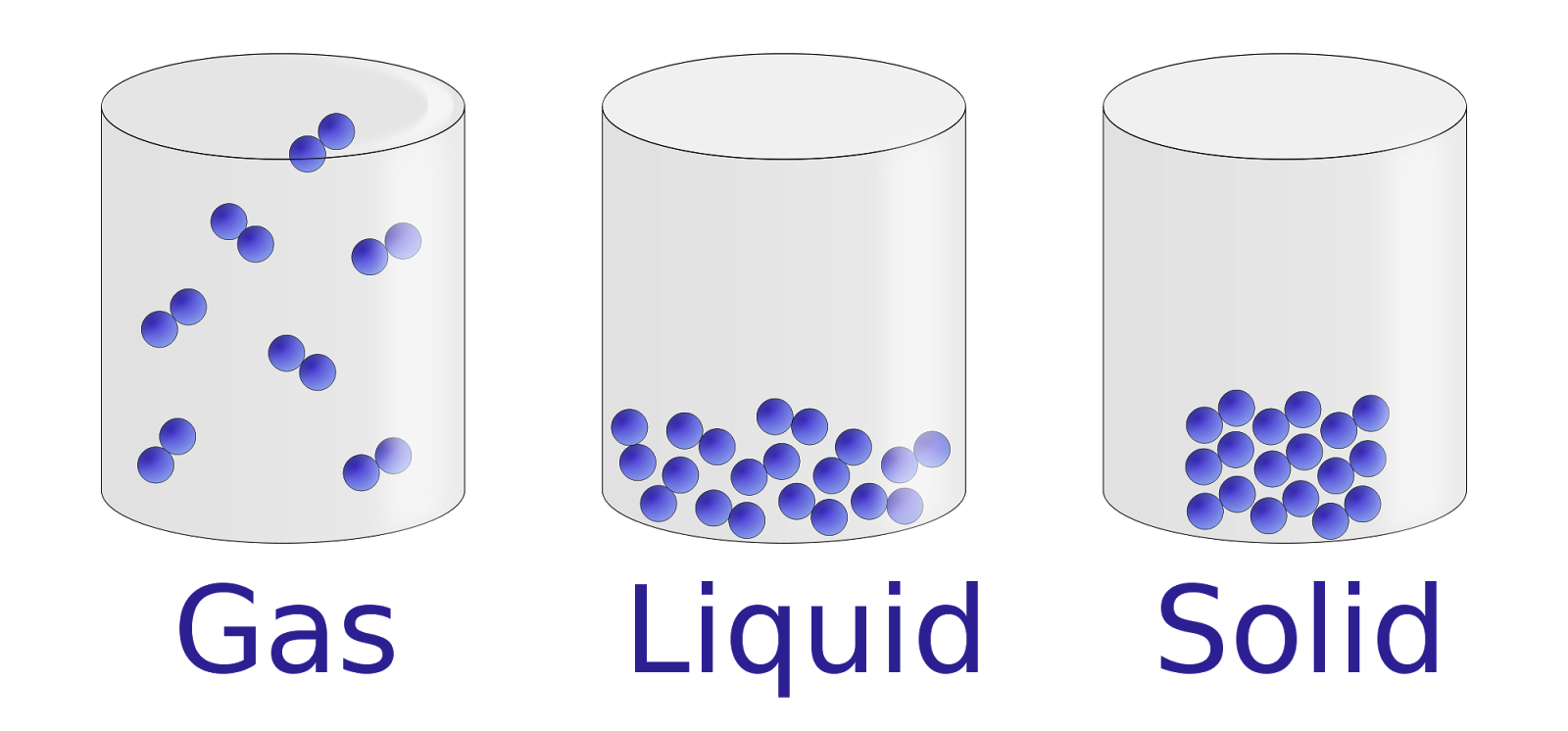
You’ve probably seen it a thousand times. A textbook page or a digital infographic showing three boxes. One is packed with neat rows of marbles. Another has marbles rolling over each other. The last one shows a few lonely dots flying around like caffeinated gnats. This classic image solid liquid gas representation is the backbone of how we understand the world, yet honestly, it’s a bit of a lie. We use these visuals because the real thing is too fast, too small, and too chaotic for our brains to track without help.
Matter is messy.
✨ Don't miss: Mac OS X Download El Capitan: How to Safely Get it Without Breaking Your Mac
In a solid, things are rigid. We think of ice or iron. In a liquid, things flow, like your morning coffee. Gases are the invisible stuff—steam, oxygen, the smell of burnt toast. But when you look at an image solid liquid gas diagram, you're looking at the "Kinetic Molecular Theory" in its most basic, stripped-down form. It’s the starting point for everything from HVAC engineering to understanding how stars are born.
The Problem With the Marbles
Most people think those little circles in the diagrams are just "stuff." They’re atoms or molecules, obviously. But the way an image solid liquid gas layout presents them usually misses the most important part: the energy.
Solid particles aren't just sitting there. They’re vibrating. Even in a frozen block of steel, those atoms are shaking like they’ve had too much espresso, just trapped in a cage of chemical bonds. When we look at a static image, we lose that sense of violence. If you could actually see the molecules in a solid, it wouldn't look like a neat grid of pool balls; it would look like a frantic, shimmering crowd trapped in a packed subway car.
Why liquids are the hardest to draw
If solids are easy (neat rows) and gases are easy (scattered dots), liquids are the middle child of science visuals. They're weird. They have a fixed volume but no fixed shape. In a standard image solid liquid gas comparison, the liquid section usually looks like a disorganized pile.
Actually, liquids are held together by "intermolecular forces" that are just strong enough to keep everyone close but weak enough to let them slide around. It’s like a mosh pit at a concert. Everyone is touching, but everyone is moving. This fluidity is why hydraulics work. You can't compress a liquid easily because the particles are already touching, but you can move them. That’s why your car brakes work when you step on the pedal.
Moving Beyond the Three Basics
Here is the thing. The world isn't just three states.
If you’re looking at an image solid liquid gas chart in 2026, you're likely missing plasma. Or Bose-Einstein condensates. Or supercritical fluids. We stick to the "Big Three" because that's what we interact with every day. You drink water, you breathe air, you sit on a chair.
✨ Don't miss: Conway’s Game of Life: Why a 50-Year-Old Math Experiment is Still Breaking Our Brains
But think about a candle flame. Is it a gas? Sorta. It’s actually partially ionized gas, which starts leaning into plasma territory. Or think about Oobleck—that cornstarch and water mixture kids play with. It’s a "non-Newtonian fluid." If you hit it hard, it acts like a solid. If you hold it gently, it runs like a liquid. A standard image solid liquid gas diagram can't really explain why Oobleck does that because the diagram assumes everything follows the rules.
The energy jump
Phase changes are where the real magic happens. It’s not just about the "look" of the particles. It’s about the "Latent Heat."
When you see an image solid liquid gas transition, like ice melting into water, the temperature actually stays the same for a while. You’re pumping heat into the ice, but the thermometer doesn't move. Why? Because all that energy is being used as "molecular wire cutters." It’s literally snapping the rigid bonds of the solid to turn it into a liquid.
- Energy goes in.
- Bonds break.
- Movement increases.
- Phase changes.
It’s a brutal, energetic process.
Digital Tools and New Visuals
We aren't stuck with 2D drawings anymore. Technology has changed how we visualize these states. Modern simulations use "Molecular Dynamics" (MD) to show how these particles actually behave.
If you search for a high-quality image solid liquid gas today, you’re likely to find 3D renders that account for Van der Waals forces. These aren't just for school kids. Engineers at companies like Boeing or SpaceX use these visualizations to see how fuels behave in zero gravity or how heat shields handle the transition from solid to... well, melting.
What the Diagrams Get Wrong About Space
In a typical image solid liquid gas visual, the gas particles have plenty of room. In reality, the "Mean Free Path" (the distance a particle travels before hitting another one) in a gas at room temperature is tiny. But in the vacuum of space? A molecule might travel miles before it bumps into a friend.
Also, we often forget about pressure. We focus so much on temperature that we ignore that you can turn a gas into a liquid just by squeezing it hard enough. That’s how the propane in your backyard grill stays liquid inside the tank. It’s "starving" for space. The moment you open the valve, it expands back into its gas form because the pressure is gone.
📖 Related: Where Am I Now: Why Your Phone Sometimes Gets Your Location Wrong
Real-world applications of phase knowledge
- Cryogenics: Using liquid nitrogen to keep things cold depends on the massive energy jump between liquid and gas.
- Aerosols: Your hairspray or spray paint relies on a pressurized gas pushing a liquid out of a nozzle.
- Refrigeration: Your fridge is basically a machine that constantly forces a substance to change between liquid and gas to move heat from the inside to the outside.
Actionable Insights for Using These Visuals
If you are a student, a teacher, or just someone trying to explain how the world works, don't just look at a static image solid liquid gas and stop there.
Watch a simulation. Go to a site like PhET Interactive Simulations (from the University of Colorado Boulder). Use their "States of Matter" tool. It lets you actually "pump" heat into a container and watch the atoms freak out. It’s way better than a drawing.
Think about the "In-Betweens." Next time you see a cloud, realize it’s not just gas. It’s actually tiny liquid water droplets or solid ice crystals suspended in a gas. It’s a mixture. Most things in the real world are.
Check the scale. Remember that if a molecule were the size of a marble, the distance between gas molecules in a standard room would be several feet. Most diagrams make the gas look way too crowded just so you can see the dots on the screen.
Understand the "Why." Don't just memorize the names. Understand that everything boils down to a tug-of-war. On one side, you have "Intermolecular Forces" trying to pull everything together. On the other side, you have "Thermal Energy" trying to blow everything apart.
When energy wins, you get a gas. When the forces win, you get a solid. Liquids are the messy, beautiful stalemate in the middle.