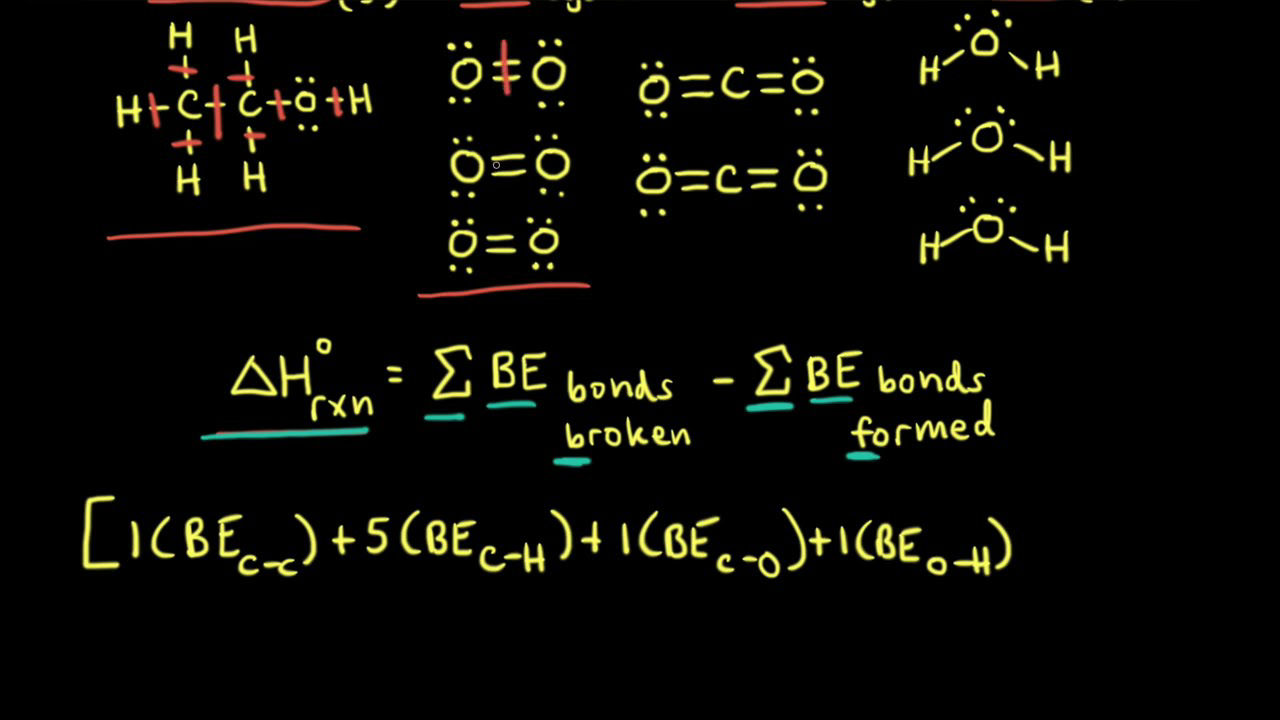You're standing over a calorimeter. Maybe it's a fancy high-tech one, or maybe it’s just two Styrofoam cups nested together in a freshman chem lab. The thermometer is flickering, the water is heating up, and you realize you have to do the math. Again. Chemistry is beautiful until it becomes a spreadsheet. That is exactly why using an enthalpy of reaction calculator isn't just "cheating"—it’s actually about getting the science right without the soul-crushing arithmetic errors that ruin a perfectly good experiment.
Enthalpy is a weird concept to wrap your head around at first. It’s not just "heat." It’s the total heat content of a system at constant pressure. Think of it as the energy bank account of a chemical reaction. Some reactions, like burning methane on your stove, are big spenders—they dump energy into the surroundings (exothermic). Others, like those instant cold packs you use for a sprained ankle, are savers—they suck energy in (endothermic). An enthalpy of reaction calculator basically tells you exactly how much that bank balance changed when the atoms finished their dance.
The Messy Reality of Bond Enthalpy
Calculating this stuff by hand usually involves Hess’s Law or bond enthalpies. If you go the bond enthalpy route, you’re basically looking at the energy required to snap every single bond in the reactants and then subtracting the energy released when the new bonds form in the products.
It sounds simple. It’s not.
Imagine you're dealing with something like the combustion of propane. You have to count every C-H bond and every C-C bond. If you miss one single bond in your tally, your whole $\Delta H$ is garbage. An enthalpy of reaction calculator automates this tedious counting. You punch in the molecules, and the software references a database of standard values—like those found in the CRC Handbook of Chemistry and Physics—to give you a number that actually makes sense.
Standard values are usually measured at 298.15 K (25°C) and 1 atm of pressure. If your lab is freezing cold or you're high up in the mountains of Colorado, your real-world results will deviate. That's the first thing people get wrong: they expect the calculator to be a magic crystal ball. It’s a reference tool. It tells you what should happen in a perfect world.
Why Hess's Law Still Matters
Sometimes you can't just count bonds. Some reactions are too dangerous or too slow to measure directly. This is where Germain Hess, a Swiss-born Russian chemist, comes in. He figured out back in the 1800s that the total enthalpy change for a reaction is the same whether it happens in one step or ten. It’s a "state function."
If you're using a high-end enthalpy of reaction calculator, it might use the Heat of Formation ($\Delta H_f$) method. This is the "products minus reactants" rule. You take the sum of the heats of formation of all the products and subtract the sum of the heats of formation of the reactants.
👉 See also: The Facebook User Privacy Settlement Official Site: What’s Actually Happening with Your Payout
$$\Delta H_{rxn} = \sum \Delta H_f(products) - \sum \Delta H_f(reactants)$$
Simple, right? Not if you forget the stoichiometric coefficients. If your balanced equation has a "3" in front of the CO2, you have to triple that enthalpy value. Humans forget this all the time. Computers don't.
When the Math Goes Wrong
I’ve seen students spend three hours chasing a decimal point. They’ll have a reaction that is clearly boiling hot to the touch, yet their handwritten math says the reaction is endothermic. They forgot a negative sign.
In chemistry, the sign is everything.
- Negative $\Delta H$: The system lost energy. It’s hot. (Exothermic)
- Positive $\Delta H$: The system gained energy. It’s cold. (Endothermic)
A good enthalpy of reaction calculator acts as a sanity check. If you’re doing a titration or a neutralization reaction between HCl and NaOH, the calculator will scream at you (metaphorically) if your manual math doesn't result in a negative value. We know that reaction produces heat. If your paper says otherwise, trust the silicon over the graphite.
Surprising Nuances: It's Not Just About Temperature
Most people think enthalpy is just about temperature change. But there's a reason we use the symbol $H$ and not just $Q$ (heat). Enthalpy includes internal energy plus the product of pressure and volume ($H = U + PV$).
In a reaction where a solid turns into a gas, like baking soda reacting with vinegar, the system has to "push" the atmosphere out of the way to make room for the new CO2 bubbles. That takes energy! That’s work! A basic enthalpy of reaction calculator handles these P-V work considerations by using standard enthalpies of formation which already have that "pushing" energy baked into the numbers.
✨ Don't miss: Smart TV TCL 55: What Most People Get Wrong
The Problem with "Standard" Conditions
We need to talk about the "degree" symbol ($\Delta H^\circ$). That little circle means "Standard State."
Honestly, almost no one is actually working in standard state.
- Concentration: Standard state for a solute is 1.0 M. If you're using 0.1 M HCl, your enthalpy per mole might stay similar, but the total heat released will be much lower.
- Pressure: Standard state is 1 bar (about 1 atm).
- Allotropes: For elements, the standard state is the most stable form at 1 bar. For carbon, that’s graphite. If you’re somehow reacting diamonds (weird flex, but okay), your starting enthalpy is already different.
Most online tools assume you are using the most stable allotrope. If you're working with white phosphorus instead of red phosphorus, you need to be careful with which buttons you click.
Real-World Applications That Actually Matter
Why do we care? Aside from passing a mid-term?
Engineers use these calculations to design rocket engines. If you're mixing liquid hydrogen and liquid oxygen, you need to know exactly how much energy is going to be released. Too little? The rocket stays on the pad. Too much? The engine becomes a bomb. They aren't doing this on a cocktail napkin; they are using sophisticated versions of an enthalpy of reaction calculator integrated into their CAD and simulation software.
In the food industry, enthalpy helps calculate the caloric content of food. When you read that a serving of chips has 200 calories, that’s essentially a measurement of the enthalpy of combustion. They literally burn the food in a bomb calorimeter and measure the heat.
Moving Beyond the Basics
If you're looking for a tool, don't just settle for the first one that pops up on a search engine. Look for one that allows you to specify the state of matter. Water as a liquid ($H_2O(l)$) has a different heat of formation than water as a gas ($H_2O(g)$). The difference is the heat of vaporization.
$$\Delta H_f [H_2O(l)] = -285.8 kJ/mol$$
$$\Delta H_f [H_2O(g)] = -241.8 kJ/mol$$
🔗 Read more: Savannah Weather Radar: What Most People Get Wrong
That 44 kJ difference is huge! If your enthalpy of reaction calculator doesn't ask you if the water produced is steam or liquid, it's giving you a ballpark figure, not a precise one.
How to Get the Most Out of Your Calculations
To really master this, you have to stop treating the calculator like a black box.
First, balance your equation. No tool can help you if you’re trying to react one mole of propane with one mole of oxygen. It’s chemically impossible. You need five moles of $O_2$ for every one mole of $C_3H_8$.
Second, check your units. Most calculators default to kJ/mol. Some use kcal. If you mix these up, you’ll be off by a factor of 4.184. That’s the difference between a slight warm-up and a catastrophic meltdown in a high-pressure reactor.
Third, consider the environment. If you’re doing this for a real experiment, remember that the "surroundings" include the glass beaker, the air, and the thermometer itself. They all soak up some of that enthalpy. This is the heat capacity of the calorimeter ($C_{cal}$).
Actionable Steps for Your Next Lab
- Balance First: Before opening any browser tab, write out your reaction and ensure every atom is accounted for on both sides.
- Identify States: Note whether your reactants and products are solid ($s$), liquid ($l$), gas ($g$), or aqueous ($aq$).
- Run a Double-Check: Use an enthalpy of reaction calculator to find the theoretical $\Delta H$.
- Calculate % Error: Once you finish your physical experiment, compare your "real" number to the calculator's "ideal" number.
- Analyze the Gap: If your experimental value is much lower than the calculated value, look for heat loss. Did you cap the calorimeter? Was the stir bar generating friction?
The goal of using these tools isn't to avoid the science—it's to free up your brain to actually understand the science. Instead of stressing over whether $8 \times 413$ is $3304$ or $3404$, you can focus on why the molecular orbitals are shifting and what that means for the stability of the new compound you've just created. That’s where the real chemistry happens.
Stop doing the busy work. Use the tool, verify the data, and get back to the actual discovery.