You’re standing in a kitchen, and you decide to bake a cake. You mix flour, eggs, sugar, and cocoa powder. You toss that gooey mess into the oven. Thirty minutes later, you pull out something completely different: a fluffy, chocolatey sponge. In the world of chemistry, that cake is your result. But if we’re being technical, we call it the product. Basically, what is the product in chemical reaction? It’s the "after" picture. It is the substance, or group of substances, formed when chemical bonds break and reform during a specific event.
Chemistry isn't just about beakers and lab coats. It’s happening in your stomach right now. It’s happening in the engine of your car. It’s the final destination of a molecular journey.
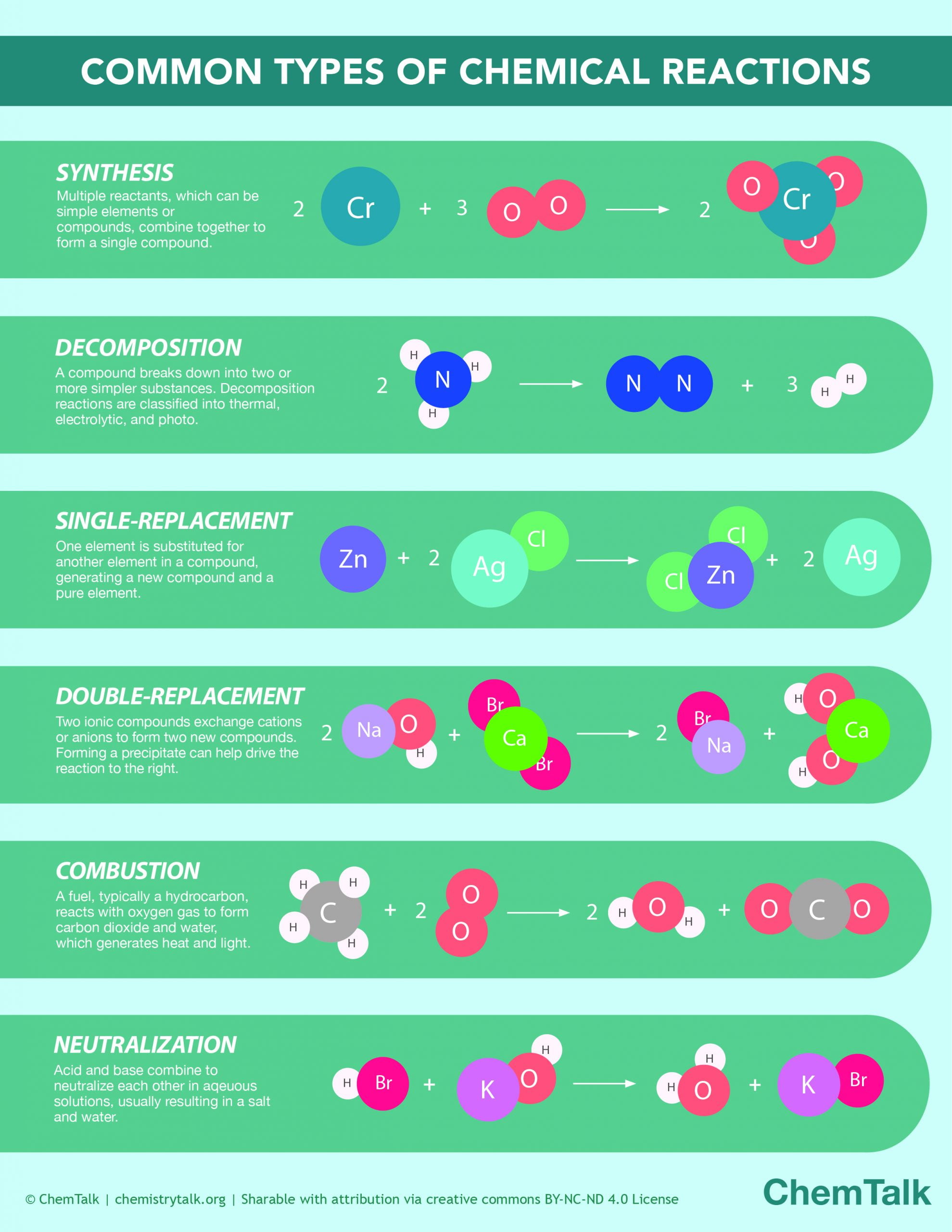
The Anatomy of a Chemical Equation
When scientists want to describe this process without writing a whole novel, they use a chemical equation. Think of it like a recipe. On the left side, you have your starting materials, known as reactants. Then there’s an arrow pointing to the right. That arrow is the "yields" sign. It’s the moment of transformation. On the right side of that arrow? That’s where you find the product.
Take a classic example: making water. You take two molecules of hydrogen gas ($2H_2$) and one molecule of oxygen gas ($O_2$). When they react, they don’t just sit next to each other. They rearrange. The result is two molecules of water ($2H_2O$). In this scenario, the water is the product. It’s a totally different beast than the gases that created it. Hydrogen is explosive. Oxygen supports combustion. But the product? It’s what we use to put out fires. That’s the magic of chemistry—the products often have entirely different physical and chemical properties than the reactants.
Identifying the Product in the Wild
You might wonder how to spot the product if you aren't looking at a textbook. Honestly, it’s usually the thing that wasn't there when you started. If you leave a piece of iron outside in the rain, it eventually turns orange and crumbly. That’s rust (iron oxide). The iron and the oxygen from the air were the reactants. The rust is the product.
In a lab setting, identifying products can be a bit more complex. Chemists use things like mass spectrometry or infrared spectroscopy to confirm what they’ve actually made. Sometimes, you get what you expected. Other times, you get "side products." These are the unwanted guests at the party—extra substances that form because the conditions weren't perfect or because the chemicals decided to take a different path.
Reactants vs. Products: The Molecular Handshake
The transition from reactant to product isn't instantaneous in the way we perceive it. It involves the Transition State. This is a high-energy, unstable middle ground where old bonds are halfway broken and new ones are halfway formed.
- Energy Input: Most reactions need a little "nudge" to get started, called activation energy.
- The Breakup: The atoms in the reactants stop holding onto each other.
- The New Relationship: Atoms find new partners, creating the product.
Law of Conservation of Mass: Why Nothing Is Lost
Here is where people sometimes get tripped up. You might look at a pile of wood burning and think the product (ash) is way smaller than the reactant (the log). You'd think matter just vanished.
🔗 Read more: The MOAB Explained: What Most People Get Wrong About the Mother of All Bombs
Nope.
Antoine Lavoisier, the "Father of Modern Chemistry," proved this back in the 18th century. He showed that mass is conserved. If you could trap all the smoke, water vapor, and carbon dioxide gas coming off that burning log and weigh it along with the ash, it would weigh exactly the same as the original log and the oxygen it consumed. The product in chemical reaction carries every single atom that was present at the start; they’re just wearing new outfits.
Types of Products You’ll Encounter
Not all products are created equal. Depending on the reaction type, the product might be a solid, a liquid, or a gas.
- Precipitates: In some liquid reactions, a solid suddenly appears and sinks to the bottom. This "cloudiness" is a solid product called a precipitate.
- Gaseous Products: If you drop an Alka-Seltzer in water, the fizzing is the production of carbon dioxide gas. The gas is the product you can see (and hear).
- Effluent: In industrial chemistry, the product might be a stream of liquid waste or a purified chemical destined for a shelf.
Why Do We Care About the Product?
In the pharmaceutical industry, the product is everything. Scientists spend years trying to figure out how to make a specific "product" (a drug molecule) with 100% purity. If the reaction produces even 1% of the wrong product, it could be toxic.
Catalysts: The Matchmakers
Sometimes, a reaction is too slow. It needs a catalyst. A catalyst is a bit like a wedding planner. It helps the reactants get together to form the product much faster, but—and this is a key point—the catalyst itself is not a product. It’s also not a reactant. It’s still there at the end, unchanged, ready to help the next set of molecules. If you see a chemical written above the arrow in an equation, that’s your catalyst. Don't mistake it for the product.
Reversible Reactions: When Products Become Reactants
Just to keep things interesting, some reactions aren't a one-way street. These are called reversible reactions. They use a double arrow ($\rightleftharpoons$).
In these cases, as soon as the products are formed, they can react with each other to turn back into the original reactants. It’s a constant tug-of-war. Eventually, they reach a state called chemical equilibrium, where the rate of the forward reaction equals the rate of the reverse reaction. In this environment, the "product" is technically whatever is on the right, but the molecules are constantly swapping roles. It’s chaotic but balanced.
💡 You might also like: What Was Invented By Benjamin Franklin: The Truth About His Weirdest Gadgets
Real-World Examples of Chemical Products
Let's look at some things you interact with daily.
Photosynthesis
Plants are the ultimate chemists. They take carbon dioxide and water (reactants) and use sunlight to turn them into glucose and oxygen.
- Reactants: $6CO_2 + 6H_2O$
- Products: $C_6H_{12}O_6$ (Sugar) + $6O_2$ (Oxygen)
Cellular Respiration
This is the reverse. Your body takes that sugar and oxygen to create energy.
- Reactants: Glucose and Oxygen
- Products: Carbon Dioxide, Water, and ATP (energy)
Vinegar and Baking Soda
The classic volcano experiment.
- Reactants: Acetic acid and Sodium bicarbonate
- Products: Sodium acetate, water, and carbon dioxide. The carbon dioxide is what makes the "lava" bubbles.
The Role of Stoichiometry
How much product can you actually make? Chemists use a math-heavy branch of science called stoichiometry to figure this out. If you have five grams of Reactant A, how many grams of Product B will you get?
You have to consider the limiting reactant. Imagine you’re making ham sandwiches. You have ten slices of bread and two slices of ham. Even though you have plenty of bread, you can only make two sandwiches because you ran out of ham. The ham is the limiting reactant. In chemistry, the amount of product you get is always limited by whichever reactant runs out first.
How to Determine the Product Yourself
If you’re looking at a reaction and trying to figure out what the product will be, follow these steps:
📖 Related: When were iPhones invented and why the answer is actually complicated
Identify the reaction type.
Is it synthesis (two things becoming one)? Decomposition (one thing breaking apart)? Combustion (something reacting with oxygen)?
Check the charges.
If it’s an ionic reaction, make sure the positive and negative charges in your new product balance out to zero. Nature loves balance.
Balance the equation.
Remember Lavoisier? You can't have three carbons on the left and only one on the right. You have to add coefficients (those big numbers in front) to make sure the atom count matches on both sides of the arrow.
Surprising Truths About Products
One of the most mind-blowing things about chemical products is how they can be safer or more dangerous than their parts. Chlorine gas is a deadly weapon used in WWI. Sodium is a metal that explodes if it touches water. Put them together in a chemical reaction? You get sodium chloride. Table salt. You put it on your popcorn.
The product is a brand-new identity.
Common Misconceptions
People often think that the product is just a "mixture" of the reactants. It’s not. A mixture is like sand and salt stirred together—you can still see both. A product is the result of a chemical change. The original substances are gone. Their atoms have been repurposed.
Another mistake is thinking that heat or light are products. While a reaction can release energy (exothermic) or absorb it (endothermic), heat and light aren't substances. They don't have mass. They are "byproducts" in terms of energy, but when we talk about the "product in chemical reaction," we are specifically referring to the new matter formed.
Actionable Insights for Students and Enthusiasts
To truly master the concept of products in chemistry, stop just looking at the letters and start visualizing the atoms.
- Practice Balancing: Take five simple equations today and balance them. It’s the only way to internalize the law of conservation of mass.
- Watch the Signs: Look for color changes, temperature shifts, or gas bubbles in everyday life. That is the physical manifestation of a product being born.
- Use Tools: If you're stuck on a complex organic reaction, use software like ChemDraw or online simulators to see how molecules "flip" to become products.
- Predict the Outcome: Before looking at the right side of an equation, try to guess the product based on the reactants' valence electrons.
Understanding the product is the key to understanding how the physical world is built, destroyed, and recycled. Whether it's the plastic in your phone or the air you're breathing, everything around you is a product of a reaction that happened somewhere, sometime.