You’re breathing right now. It's automatic. You don't even think about it. But if you stop for a second and wonder what is oxygen made of, the answer isn't just "air." Honestly, air is mostly nitrogen anyway, which is a weird thought when you realize oxygen is the thing actually keeping your cells from shutting down.
Oxygen is an element. That sounds like a textbook answer, but it means you can't break it down into something else using a chemical reaction. If you take a gold bar and chop it into tiny pieces, you eventually get a gold atom. Oxygen is the same way. At its core, it is a collection of subatomic particles—protons, neutrons, and electrons—organized in a very specific, high-energy dance that makes life on Earth possible.
The Atomic Recipe for Oxygen
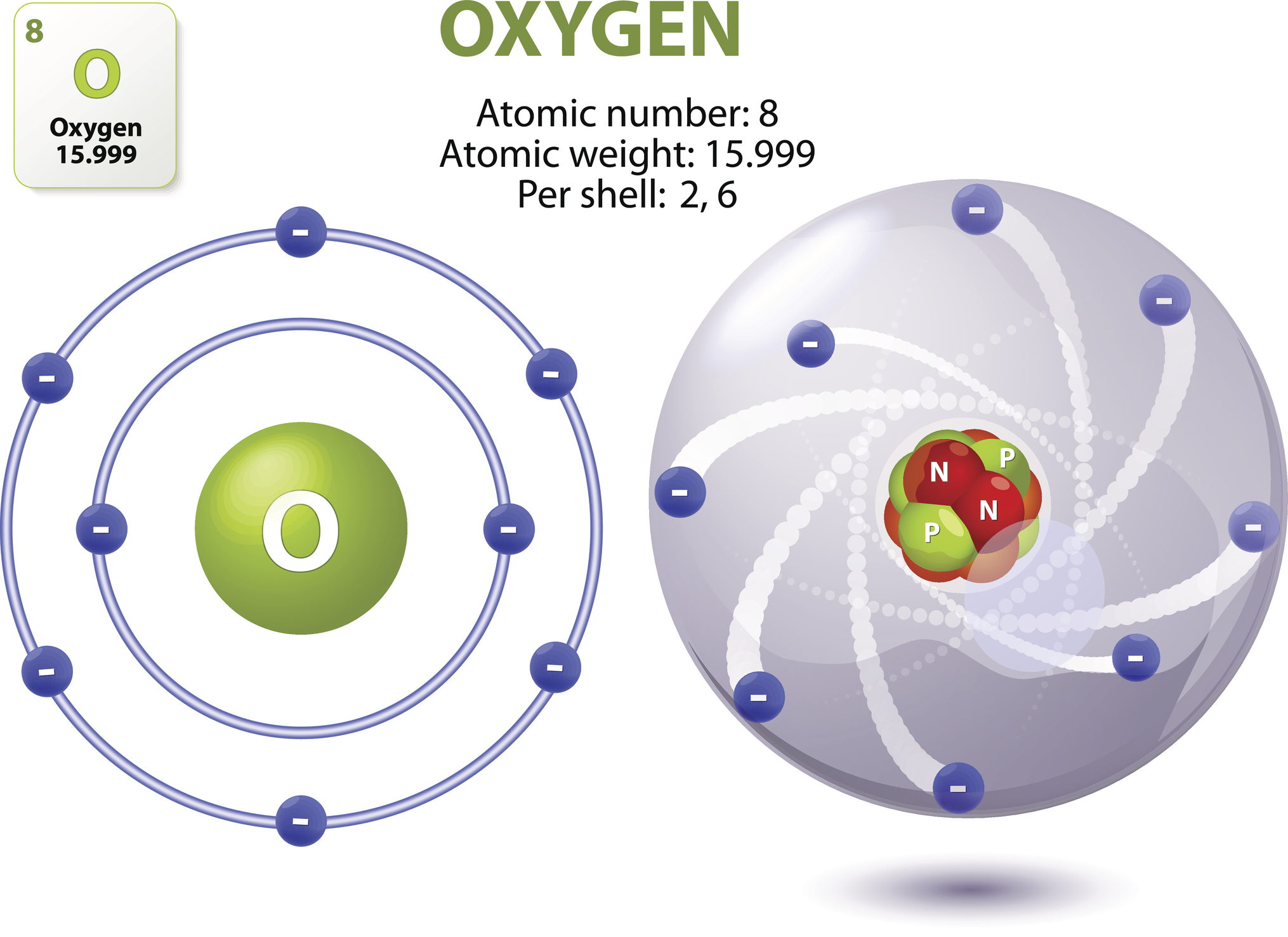
To understand what oxygen is made of, you have to look at the "ingredients" inside a single atom. Every oxygen atom in the universe, whether it’s in a scuba tank or trapped inside a rust spot on an old truck, has exactly eight protons. This is its identity. If it had seven, it would be nitrogen. If it had nine, it would be fluorine.
Protons are the heavy, positively charged bits in the center, called the nucleus. Sitting right next to them are neutrons. Most oxygen atoms you encounter have eight neutrons, making the total mass 16. Scientists call this Oxygen-16. It’s the "standard" version. However, nature likes to mix things up. About 0.2% of oxygen atoms have ten neutrons, known as Oxygen-18. It’s slightly heavier, and climate scientists actually use the ratio of these different "isotopes" in ancient ice cores to figure out how hot or cold the Earth was thousands of years ago.
👉 See also: The Truth About Every Casio Piano Keyboard 88 Keys: Why Pros Actually Use Them
Then you have the electrons. There are eight of them, buzzing around the nucleus in shells. These are the social butterflies of the atom. Because the outer shell of an oxygen atom isn't full, it is incredibly "hungry" to grab electrons from other elements. This is why oxygen is so reactive. It’s why wood burns and why iron turns into orange flakes. It’s basically the universe’s most aggressive negotiator.
Where Does All This Oxygen Actually Come From?
It wasn't always here. Early Earth was a suffocating mess of methane and carbon dioxide. If you stepped out of a time machine 3.5 billion years ago, you'd drop dead instantly.
So, what is oxygen made of in terms of its cosmic history? It was forged in the bellies of massive stars. Through a process called nuclear fusion, stars smash helium atoms together to create heavier elements. When these stars eventually explode in a supernova, they spray oxygen across the galaxy. Every breath you take contains atoms that were once inside a dying star.
✨ Don't miss: iPhone 15 size in inches: What Apple’s Specs Don't Tell You About the Feel
On Earth, we owe our oxygen supply to tiny organisms. About 2.4 billion years ago, something called the Great Oxidation Event happened. Cyanobacteria—basically pond scum—figured out how to use sunlight to turn water and CO2 into food. The "waste product" of this process was oxygen. They pooped out so much oxygen that it changed the entire chemistry of the planet. It killed off most of the existing life that couldn't handle the "toxic" gas, but it cleared the way for us.
The Different "Flavors" of Oxygen
We usually talk about oxygen as $O_2$. This is the stuff we breathe. Two oxygen atoms holding hands. It's stable and travels through your bloodstream easily.
But there is another version: Ozone ($O_3$). This is three oxygen atoms shoved together. While $O_2$ is life-giving, $O_3$ is a bit of a Jekyll and Hyde. High up in the stratosphere, it protects us from getting fried by UV rays. But at ground level, in smog, it’s a lung irritant. It’s the same "stuff," just arranged differently.
🔗 Read more: Finding Your Way to the Apple Store Freehold Mall Freehold NJ: Tips From a Local
Then you have liquid oxygen. If you cool oxygen down to $-183^\circ C$ ($-297^\circ F$), it turns into a beautiful, pale blue liquid. It's also terrifyingly dangerous. In its liquid form, it is a concentrated oxidizer. Space companies like SpaceX and NASA use it as propellant. When you see a Falcon 9 rocket screaming into the sky, that massive white cloud isn't just smoke; a lot of it is the result of liquid oxygen reacting with fuel to create thrust. It’s the same element in your lungs, just compressed into a violent, high-energy state.
Why It Matters: Beyond Just Breathing
Understanding what oxygen is made of helps explain why it binds to so many things. It makes up nearly half of the Earth's crust by mass. It isn't just "in the air"—it is in the rocks, the sand, and the soil.
When oxygen meets hydrogen, you get water ($H_2O$). When it meets iron, you get rust. In your body, oxygen's job is to sit at the end of a long chain of chemical reactions in your mitochondria. It acts as the "trash collector" for electrons, allowing your body to burn sugar for energy. Without that specific atomic structure—those eight protons and eight electrons—this process would fail, and your "battery" would run out in minutes.
Practical Steps for Better Oxygen Levels
While we can't change the atomic makeup of oxygen, we can change how we interact with it. Most people are "shallow breathers," only using the top third of their lungs.
- Practice Diaphragmatic Breathing: Also known as belly breathing. This allows for better gas exchange in the lower lobes of the lungs, where blood flow is highest.
- Monitor Indoor Air Quality: CO2 buildup in offices or bedrooms can make you feel groggy. It isn't always a lack of oxygen, but an excess of carbon dioxide that makes the "mix" wrong. Open a window for 10 minutes a day.
- Invest in Plants: While a single spider plant won't replace a forest, having greenery indoors can slightly improve local air quality and humidity, making oxygen intake feel "fresher."
- Check Your Iron: Since oxygen is carried by hemoglobin (which contains iron), being anemic means your body can't move oxygen effectively, regardless of how much you breathe. If you're constantly tired, check your ferritin levels.
Oxygen is a paradox. It’s a byproduct of ancient bacteria, a fuel for stars, and a corrosive gas that eventually breaks everything down. But at its simplest level, it’s just eight protons, doing exactly what they’ve done since the beginning of time.