You probably remember the poster of the periodic table hanging in your middle school classroom. It was colorful, intimidating, and full of single elements like Hydrogen, Gold, and Oxygen. But here’s the thing: the world isn't really made of those lonely elements. Almost everything you touch, breathe, or eat is actually a combination. So, what is a compound in science exactly? Honestly, it’s just nature’s way of leveling up.
A compound happens when two or more different elements decide to hook up chemically. They aren't just hanging out in the same room like a bowl of mixed nuts. They are bonded. Fixed. Changed. When these elements bond, they lose their individual "personalities" and become something entirely new with a unique set of rules.
It’s kind of wild when you think about it. Take salt, for example. You’ve got Sodium, which is a soft metal that explodes if it touches water. Then you’ve got Chlorine, which is a deadly green gas used in chemical warfare. You put them together and what do you get? Something you put on your popcorn. That’s the magic of a chemical compound.
The Chemistry of Connection: How Compounds Actually Form
To really get what a compound is, you have to look at the "glue" holding things together. This isn't just a physical mixture. If you mix sand and salt, you can eventually pick the salt out with enough patience. That's a mixture. But in a compound, the elements are stuck together by chemical bonds.
There are basically two main ways this happens. First, you’ve got ionic bonds. This is like a game of give and take. One atom basically says, "Hey, I have an extra electron I don't want," and another atom says, "Perfect, I need one." They swap, they become oppositely charged, and they snap together like magnets. Think of Sodium Chloride ($NaCl$).
Then there are covalent bonds. This is more like a roommate situation where everyone agrees to share. Atoms huddle close and share electrons to stay stable. Water ($H_2O$) is the classic example here. Two hydrogens and one oxygen sharing the load. It's the most famous compound on Earth, and without that specific covalent arrangement, life just... wouldn't happen.
Fixed Proportions are a Big Deal
One thing people often miss is the "Law of Definite Proportions." This was popularized by French chemist Joseph Proust back in the late 1700s. Basically, he realized that a compound is always made of the same elements in the exact same ratio by mass.
🔗 Read more: Why Did Google Call My S25 Ultra an S22? The Real Reason Your New Phone Looks Old Online
Water is always two parts hydrogen to one part oxygen. If you change that ratio—say, you add an extra oxygen—you don't have "different water." You have Hydrogen Peroxide ($H_2O_2$). That stuff will bleach your hair or sting a cut; you definitely don't want to chug a glass of it at dinner. The recipe for a compound is set in stone.
Why We Care About What is a Compound in Science
You might wonder why we even bother distinguishing between elements and compounds in the real world. It's because the properties change so drastically. Look at Carbon. In its elemental form, it could be the graphite in your pencil or a diamond on a ring. But when it bonds with Oxygen to form Carbon Dioxide ($CO_2$), it becomes a greenhouse gas that plants breathe and humans exhale.
Different compounds dominate different industries:
- Medicine: Almost every drug you take, from Ibuprofen to complex chemotherapy agents, is a synthetic compound designed to interact with your body’s biology.
- Tech: The Silicon chips in your phone rely on compounds like Silicon Dioxide to function as insulators.
- Environment: Understanding how Nitrogen compounds like Ammonia ($NH_3$) work is the only reason we can grow enough food to feed 8 billion people.
It’s all about the transformation.
Sorting the Mess: Organic vs. Inorganic
Scientists like to put things in boxes. When it comes to compounds, the biggest boxes are "Organic" and "Inorganic."
For a long time, people thought organic compounds could only be made by living things. We call that "vitalism." Then, in 1828, a guy named Friedrich Wöhler accidentally made Urea—a compound found in urine—out of inorganic starting materials in a lab. He basically proved that life isn't "magic"; it's just very complex chemistry.
💡 You might also like: Brain Machine Interface: What Most People Get Wrong About Merging With Computers
Generally, if it has Carbon-Hydrogen bonds, it's organic. Sugar, DNA, methane, and plastic are all organic. If it doesn't, like salt or sulfuric acid, it's inorganic. There are exceptions, of course, because science loves to be difficult (looking at you, Carbon Dioxide), but that’s the general vibe.
Common Misconceptions About Compounds
A lot of people get confused between a molecule and a compound. It's sort of a "square is a rectangle" situation. All compounds are molecules, but not all molecules are compounds.
Wait. Let me explain.
If you have two Oxygen atoms bonded together ($O_2$), that’s a molecule because it's more than one atom. But it’s not a compound because it only contains one type of element. To be a compound, you need a variety of elements. You need a "mix" at the atomic level.
Another weird thing? Compounds have totally different physical properties than their "parents."
- Color: Two clear gases can make a blue liquid.
- State: Two gases can make a solid.
- Reactivity: Two unstable elements can make something totally inert.
It’s a complete identity shift.
📖 Related: Spectrum Jacksonville North Carolina: What You’re Actually Getting
How to Identify a Compound in the Wild
If you’re looking at a substance and trying to figure out if it's a compound or just a mixture, ask yourself these questions:
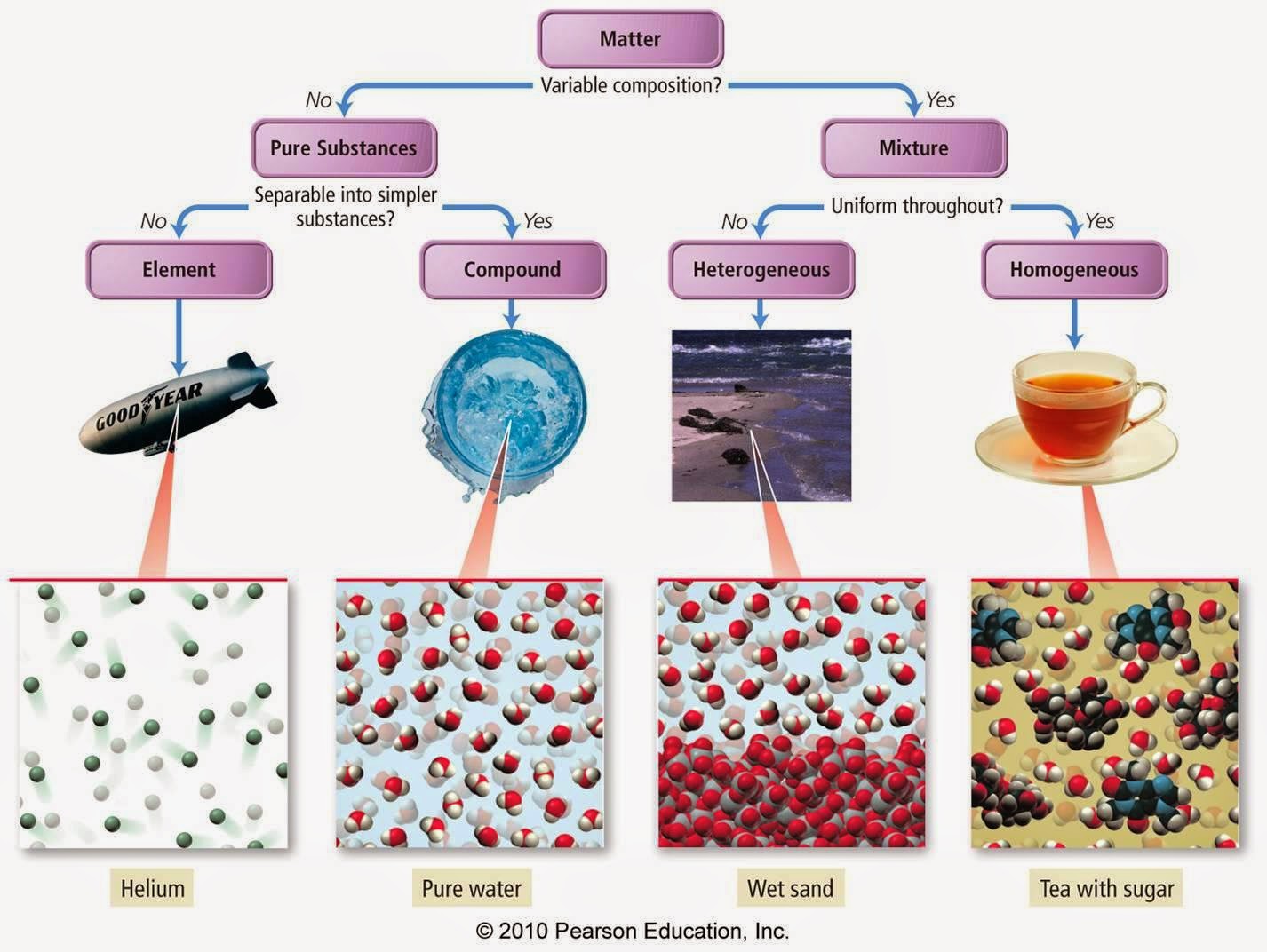
- Can I separate it easily? If you can use a filter, a magnet, or just pick it apart, it’s a mixture. If you need a massive chemical reaction or high-voltage electricity (electrolysis) to break it down, it’s a compound.
- Is it uniform? Compounds are homogeneous. Every single "piece" of water is exactly like every other "piece" of water.
- Did the properties change? If the end result looks and acts nothing like the ingredients you started with, you've likely got a compound on your hands.
Practical Steps for Mastering Chemical Literacy
Understanding what a compound is in science isn't just for passing a chemistry quiz. It’s about understanding the "ingredients list" of the universe. If you want to dive deeper, here is how you can actually apply this knowledge.
First, start reading labels on your food and household cleaners. Instead of seeing "Sodium Benzoate" as a scary chemical name, recognize it as a compound. Look up its formula ($C_7H_5NaO_2$). See those elements? Carbon, Hydrogen, Sodium, and Oxygen. They’ve bonded to create a preservative that keeps your soda from growing mold.
Second, familiarize yourself with the common "Functional Groups" in organic chemistry. If you see a name ending in "-ol," like Ethanol or Butanol, you know it’s an alcohol compound containing an Oxygen-Hydrogen ($OH$) group. This helps you predict how a substance will behave before you even touch it.
Third, if you're a student or a hobbyist, get a molecular modeling kit. Physically snapping atoms together to form a compound helps your brain visualize the 3D geometry. You’ll see why a water molecule is "bent" and why that shape makes it "sticky" enough to form raindrops.
Finally, use digital resources like the PubChem database. It’s a massive, free library of millions of compounds. You can search for any chemical name and see its structure, its dangers, and its uses. It’s the ultimate "cheat sheet" for the material world.
Everything you see is a puzzle of elements. Once you understand how they click together to form compounds, the world starts looking a lot less like a random mess and a lot more like a very complex, very cool construction project.
Next Steps for Deepening Your Understanding:
- Download a Periodic Table App: Use one that lists "common compounds" for each element. It helps bridge the gap between the element and its real-world uses.
- Perform a Simple Separation Experiment: Mix iron filings and sulfur. Use a magnet to pull the iron out (Mixture). Then, research what happens when you heat them together to form Iron Sulfide (Compound). The magnet won't work anymore.
- Study Nomenclature: Learn the basic naming rules (like why some end in "-ide" and others in "-ate"). This is the "language" of compounds and makes reading scientific papers much easier.