You’ve likely seen the smooth, white cubes of table salt a thousand times. But if you zoom in—past the kitchen table, past the grain itself, down to the atomic level—there’s a chaotic tug-of-war happening. Most people just say "ionic bonding" and call it a day. Honestly, though? It’s way more interesting than a single label.
When we talk about what interactions are present in solid nacl-ai (the "AI" often referring to the modern computational modeling of these structures), we are looking at a delicate balance of forces. It's like a high-stakes dance where every player is trying to get as close as possible without stepping on anyone's toes.
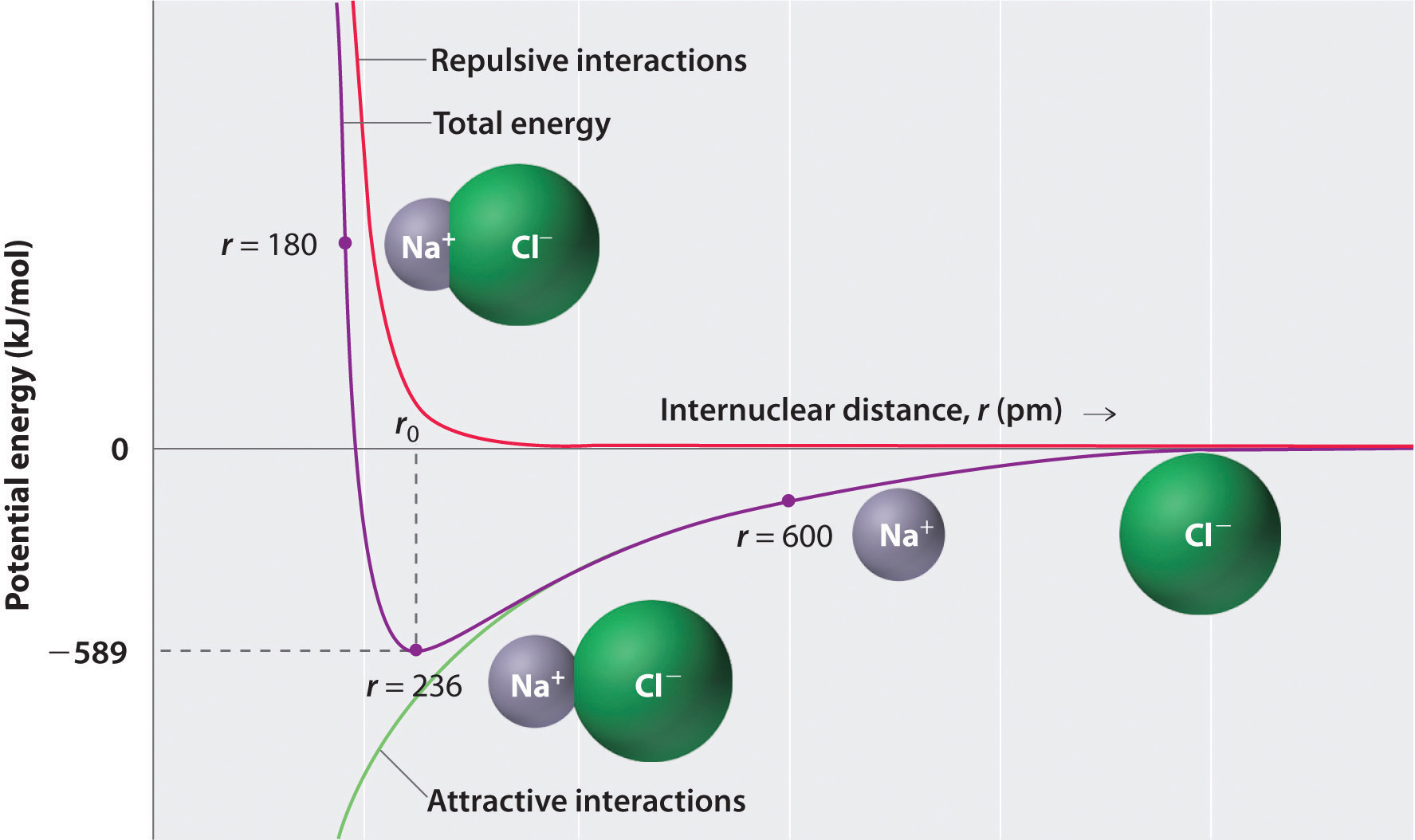
The Big One: Electrostatic (Coulombic) Attraction
At its heart, a salt crystal is held together by the same logic that makes your hair stick to a balloon. Opposites attract. In the case of NaCl, you have a sodium cation ($Na^+$) and a chloride anion ($Cl^-$).
The sodium atom basically hands over an electron to the chlorine. This isn't a "shared" arrangement like you see in water or carbon dioxide. It’s a total transfer. Because the sodium is now positive and the chlorine is negative, they are locked in a permanent embrace. This is the Coulombic interaction. It’s incredibly strong, which is why your salt doesn't just melt when you leave it on the counter; you’d need to crank the heat up to about 801°C to break those bonds.
📖 Related: How to Change an iPhone Ringtone Without Losing Your Mind
The Force Nobody Mentions: Pauli Repulsion
If opposite charges attract, why don't the ions just collapse into each other? Why is there space between them at all?
This is where things get "kinda" crowded. As the $Na^+$ and $Cl^-$ ions get closer, their electron clouds start to overlap. According to the Pauli Exclusion Principle, two electrons can't occupy the same state. When those clouds touch, a massive repulsive force kicks in. Scientists often model this using the Born-Mayer potential. Think of it like two magnets: they want to click together, but if you put a piece of rubber between them, they can only get so close.
Why the Geometry Matters (The Madelung Constant)
In a solid crystal, a sodium ion isn't just attracted to one chloride ion. It’s surrounded by six of them in a 3D grid.
This creates a complex web of interactions:
- Primary Attraction: Between a $Na^+$ and its 6 immediate $Cl^-$ neighbors.
- Secondary Repulsion: Between that $Na^+$ and the next layer of $Na^+$ ions further out.
- Tertiary Attraction: Between that $Na^+$ and the $Cl^-$ ions even further away.
The sum of all these attractions and repulsions across the entire infinite lattice is represented by something called the Madelung Constant. For the NaCl structure, this value is approximately 1.748. Without this constant, our math for calculating the energy of a salt crystal would be totally wrong.
Van der Waals: The Weakest Link
Believe it or not, even in a rock-solid ionic crystal, there are "intermolecular" forces at play. London dispersion forces (a type of van der Waals interaction) exist between the ions.
They are tiny.
Compared to the massive electrostatic pull, they’re almost negligible—accounting for maybe 1-2% of the total lattice energy. But "almost" isn't "zero." When researchers use AI and advanced simulations to predict how salt behaves under extreme pressure (like deep inside a planet), they have to account for these tiny flickers of moving electrons.
Specific Evidence from Computational Models
In modern "NaCl-AI" research—where we use machine learning to simulate crystal growth—we see that the stability of the lattice depends on the Lattice Energy.
The formula usually looks something like this:
$$U = \frac{N_A M z^+ z^- e^2}{4 \pi \epsilon_0 r_0} \left(1 - \frac{\rho}{r_0}\right)$$
This equation (the Born-Mayer equation) proves that the energy isn't just about the attraction. That little $(1 - \rho/r_0)$ part at the end is the correction for the repulsion we talked about earlier. If we didn't have that repulsion, the math would suggest the crystal should have infinite density, which is obviously impossible.
Real-World Takeaways
Knowing these interactions isn't just for passing a chemistry quiz. It explains why salt is brittle. If you hit a salt crystal with a hammer, the layers shift. Suddenly, $Na^+$ ions are lined up next to other $Na^+$ ions. The massive electrostatic attraction turns into massive repulsion instantly, and the crystal shatters along a clean line.
If you're looking to dive deeper into material science or chemistry simulations, keep these steps in mind:
- Focus on the Lattice Energy: It's the ultimate "score" of how stable a solid is.
- Account for Repulsion: Never assume attraction is the only force at play.
- Use the Madelung Constant: It's the key to moving from a single pair of atoms to a 3D solid.
- Consider Environmental Factors: In the real world, things like humidity can introduce ion-dipole interactions that start to dissolve the crystal from the outside in.