Think of your body as a massive, high-speed library. Every second, millions of books are being copied, edited, and moved around. But these aren't paper books. They’re your genetic code. If you zoom in past the organs, past the cells, and right into the nucleus, you find the building blocks. People always talk about DNA like it’s this mystical spiral staircase, but they rarely mention the actual bricks. Those bricks are nucleotides. So, what does a nucleotide consist of? It’s not just one thing. It’s a trio. A tiny, chemical trifecta that holds the blueprint for every single thing you are—from your height to that weird way you sneeze.
Honestly, it’s kind of wild how consistent nature is. Whether you’re a blue whale, a blade of grass, or a human being, the basic anatomy of a nucleotide stays remarkably similar. You’ve got three specific parts hooked together.
The Three-Part Anatomy: Sugar, Phosphate, and a Base
At its simplest, every single nucleotide is made of a sugar molecule, a phosphate group, and a nitrogenous base. That’s the "recipe." If you miss one, the whole thing falls apart. The sugar and the phosphate are like the structural backbone. They’re the "sides" of the ladder. They don’t change much. But the base? That’s where the information lives. That’s the "code."
The Pentose Sugar: The Centerpiece
The sugar in a nucleotide is a five-carbon sugar, which scientists call a pentose. Depending on whether you’re looking at DNA or RNA, this sugar changes slightly. In DNA, it’s deoxyribose. In RNA, it’s ribose.
What’s the difference? One oxygen atom. That’s it. Deoxyribose is missing an oxygen-hydrogen (hydroxyl) group at the 2' carbon. It sounds like a tiny detail, but it’s the reason DNA is stable enough to last for thousands of years in a fossil, while RNA is fragile and breaks down easily. Without that one missing oxygen, your genetic code would be way too reactive. It would basically self-destruct.
The Phosphate Group: The Connector
Then you have the phosphate group. This is one phosphorus atom bonded to four oxygen atoms. Its main job is to link the sugar of one nucleotide to the sugar of the next. This creates a "sugar-phosphate backbone." It’s incredibly strong. Because the phosphate group is negatively charged, it gives DNA an overall negative charge. This is actually why forensic scientists can use gel electrophoresis to pull DNA through a gel using electricity—the DNA moves toward the positive pole because of those phosphates.
The Nitrogenous Base: The Real Secret Sauce
Finally, we get to the nitrogenous base. This is the part that actually "talks." In DNA, there are four types:
- Adenine (A)
- Guanine (G)
- Cytosine (C)
- Thymine (T)
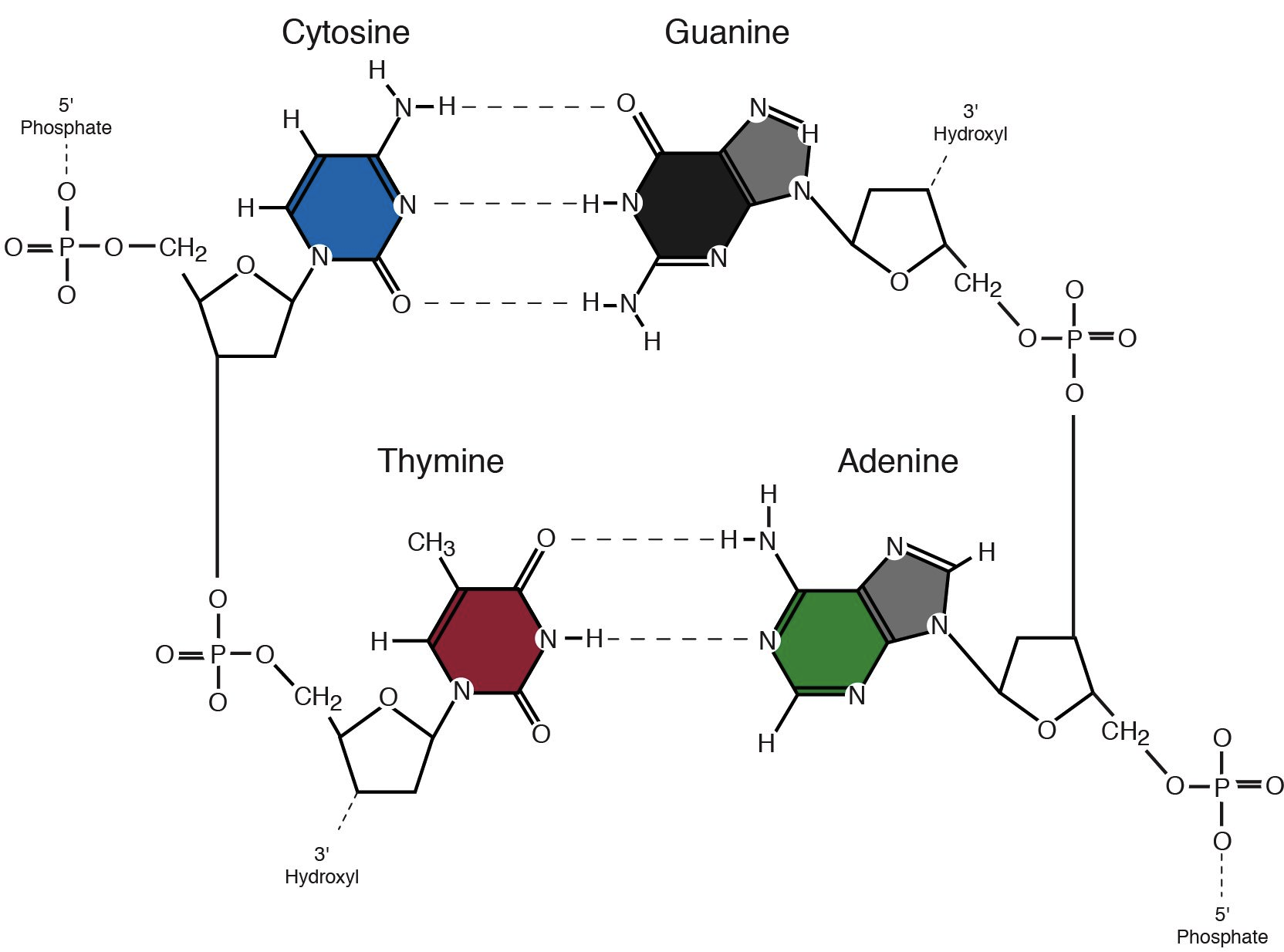
In RNA, Thymine gets swapped out for Uracil (U). These bases are the letters of your genetic alphabet. When you hear about scientists "sequencing" a genome, they’re just reading the order of these bases.
Purines vs. Pyrimidines: Why Shape Matters
Nature is obsessed with geometry. You can’t just shove any base next to another one. They have to fit. This is why we categorize them into two groups: Purines and Pyrimidines.
Adenine and Guanine are Purines. They have a double-ring structure. They’re the "big" bases. Cytosine, Thymine, and Uracil are Pyrimidines. They only have a single ring. They’re smaller.
Here is the kicker: a Purine must always pair with a Pyrimidine. A always pairs with T (or U), and C always pairs with G. This is known as Chargaff’s Rule, named after Erwin Chargaff, the biochemist who figured out the ratios were always equal. If you tried to pair two purines together, the DNA ladder would bulge out. Two pyrimidines? It would pinch in. To keep that perfect, iconic double-helix shape, the "steps" of the ladder have to be exactly the same width.
How These Parts Actually Stick Together
It’s one thing to know the parts, but how do they stay joined? It’s all about covalent bonds. Specifically, the phosphate group of one nucleotide attaches to the 3' carbon of the sugar on the next nucleotide. This is called a phosphodiester bond.
The bases, however, don't use these strong "permanent" bonds to connect to each other across the middle of the ladder. They use hydrogen bonds. These are weaker. Think of it like a zipper. You want the sides of the zipper to be made of strong fabric (covalent bonds), but you want the teeth to be able to pull apart (hydrogen bonds) so you can read the information or copy it. If the bases were glued together permanently, your cells could never "unzip" the DNA to make proteins.
ATP: The Nucleotide Nobody Recognizes
Most people think nucleotides are just for DNA. That's a huge misconception. One of the most famous molecules in biology is actually a nucleotide in disguise: ATP (Adenosine Triphosphate).
You’ve probably heard it called the "powerhouse currency" of the cell. Look at the name. Adenosine is the base (Adenine) plus the sugar (Ribose). The "Triphosphate" just means it has three phosphate groups instead of one. When your body needs energy to move a muscle or think a thought, it snaps off one of those phosphates. That release of energy is what keeps you alive. It’s kind of beautiful—the same basic structure that stores your blue-eye-color gene also provides the literal spark of energy that lets you see.
Why This Matters for Your Health
Understanding what does a nucleotide consist of isn’t just for passing a biology quiz. It’s the foundation of modern medicine.
Take cancer treatment, for example. Some chemotherapy drugs are what we call "nucleotide analogs." They look almost exactly like a real nucleotide, but they’re slightly "broken." When a fast-growing cancer cell tries to copy its DNA, it accidentally grabs the drug instead of a real nucleotide. The "fake" brick gets stuck in the wall, the DNA chain stops growing, and the cancer cell dies because it can't finish its blueprint.
🔗 Read more: Left Brain Meaning: Why Your Analytical Half Is Often Misunderstood
Then there’s CRISPR gene editing. We’re now at a point in human history where we can go in and swap one single nitrogenous base for another. Imagine a single "C" that should have been a "G." That one tiny swap can be the difference between a healthy life and a debilitating genetic disorder like Sickle Cell Anemia or Cystic Fibrosis. By understanding the chemistry of the nucleotide, we are literally learning how to edit the software of life.
Common Misconceptions to Clear Up
I see a lot of people getting confused about the "direction" of DNA. You’ll hear scientists talk about 5' (five-prime) and 3' (three-prime) ends. This refers to the carbon atoms on the sugar molecule. Because the sugars all point in the same direction, DNA has a specific orientation. It’s like a one-way street. Polymerase, the enzyme that copies your DNA, can only move in one direction. It’s why one strand of your DNA can be copied continuously, while the other has to be built in awkward little chunks called Okazaki fragments.
Also, people often think DNA is the "only" important one. RNA is just as vital. While DNA is the master blueprint that stays safe in the "office" (the nucleus), RNA is the "work order" that goes out to the "factory floor" (the ribosomes) to actually build things.
Actionable Insights: Connecting Biology to Life
If you’re interested in the health implications of your own nucleotides, there are a few things you can actually do to support "genomic stability":
- Protect the Sugar-Phosphate Backbone: Free radicals from cigarette smoke, heavy pollutants, and excessive UV radiation can cause "strand breaks." This is literally snapping the backbone of your nucleotides. Antioxidants in your diet (like those found in berries and leafy greens) help neutralize these radicals before they can do damage.
- Folate and DNA Synthesis: Your body needs Vitamin B9 (Folate) to manufacture nitrogenous bases, especially Thymine. If you are folate deficient, your body struggles to make new nucleotides, which is why folate is so critical during pregnancy when a baby is rapidly building millions of new cells.
- Understand Your Tests: If you ever get a genetic test like 23andMe or a clinical screening, you’re looking for SNPs (Single Nucleotide Polymorphisms). These are just places where one "brick" in your sequence is different from the average person's. Most are harmless, but some can tell you how you metabolize caffeine or if you're at higher risk for certain conditions.
Knowing the components of a nucleotide is like knowing how to read the fine print of your own existence. It’s a three-part system—sugar, phosphate, base—that has remained virtually unchanged for billions of years. It’s elegant, it’s simple, and it’s the reason you’re here reading this right now.
Next time you think about your health, remember it starts at the molecular level. Keeping those nucleotides happy with the right nutrients and protection is the most fundamental self-care there is. If you want to dive deeper into how these building blocks affect your metabolism, look into the role of NADH and FADH2—they’re also modified nucleotides that handle the "electricity" in your cells. Stay curious about the small stuff; it’s what makes the big stuff possible.