You're standing in your kitchen, hovering over a bowl of flour, trying to figure out if you can just swap your measuring cups for a digital scale. We've all been told that a milliliter of water weighs exactly one gram. It’s one of those "facts" we internalize in middle school science and never question again. But here’s the kicker: water density in mL isn't actually a fixed, unchangeable constant. If you’re brewing high-end coffee or running a chemistry lab in your garage, that tiny margin of error matters more than you think.
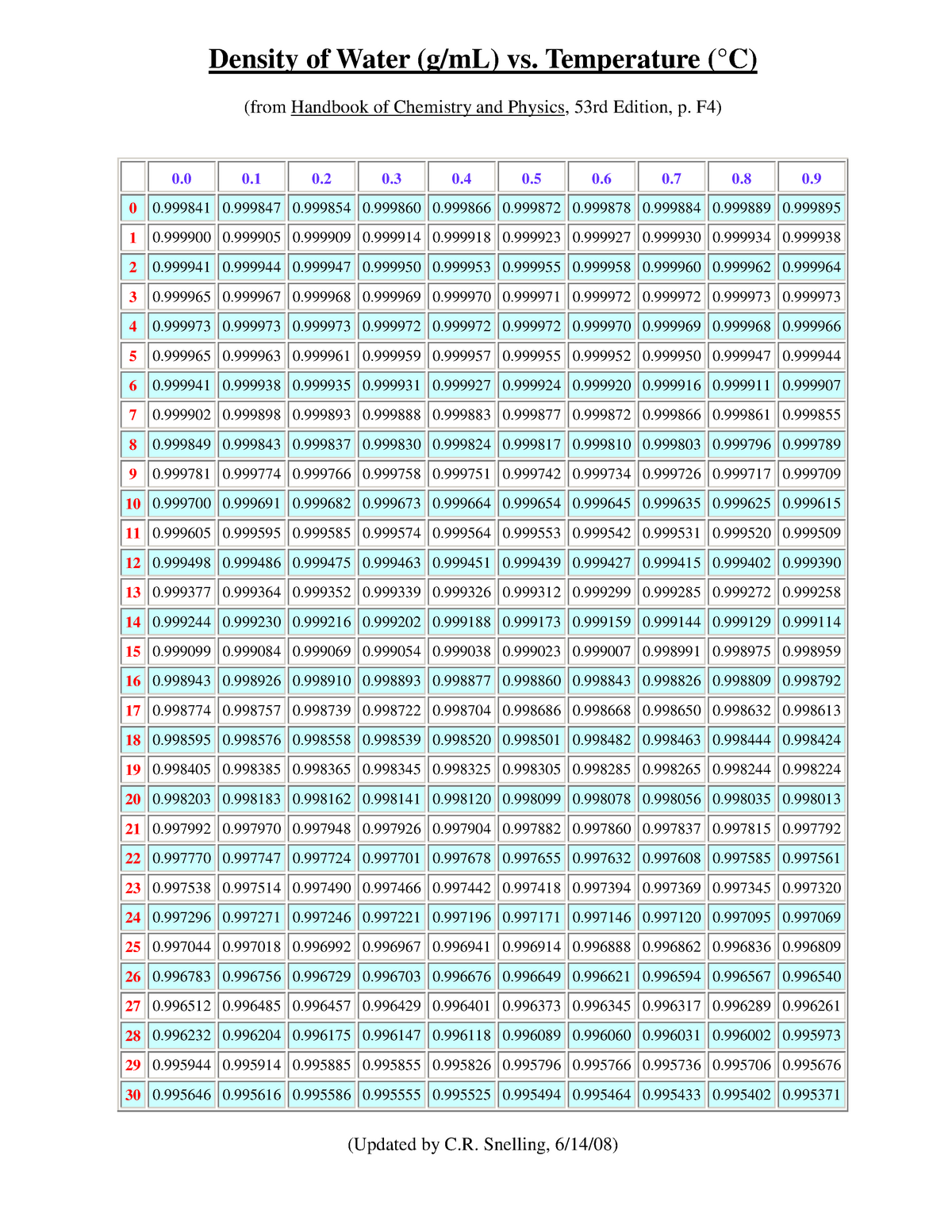
Water is weird. Honestly, it’s one of the most anomalous substances on the planet. Most things shrink and get denser as they get colder until they freeze solid. Water? It decides to be different. It hits its peak density at about 3.98°C (roughly 39°F). If you go colder than that, it actually starts expanding. That’s why ice floats. If water behaved like "normal" liquids, our lakes would freeze from the bottom up, killing everything inside.
The Math Behind the Magic (and the Mess)
Let’s talk numbers. When we talk about water density in mL, we are looking at the ratio of mass to volume. The standard definition used by the International System of Units (SI) is based on pure, distilled water at its densest point. At that specific 3.98°C mark, the density is almost exactly 1.0000 grams per milliliter ($1.0 \text{ g/mL}$).
But who drinks water at 4 degrees Celsius? Probably no one except maybe people who enjoy brain freezes. By the time that water reaches room temperature (around 20-25°C), the density drops. It’s a tiny drop—down to about $0.997 \text{ g/mL}$ or $0.998 \text{ g/mL}$.
💡 You might also like: Faux Shearling Jacket Womens: Why This Trend Actually Refuses to Die
Does it matter for your morning pasta? No.
Does it matter for precision skincare formulation or pharmaceutical compounding? Absolutely. If you are measuring out 1000 mL of water at room temperature, you’re actually getting about 997 grams. You’ve "lost" 3 grams just because the room is warm.
Why Temperature Changes Everything
When you heat water, the molecules start dancing. They move faster, they collide harder, and they push each other away. This "elbow room" increases the volume of the water without adding any mass. Same weight, more space. This is why "hot" water is less dense than "cold" water.
Consider the James Watt era of steam engines. Engineers had to account for the fact that boiling water occupies significantly more volume than cold water. In a modern context, if you're using a volumetric flask to measure water density in mL for a science project, you have to look at the calibration mark on the glass. Most laboratory glassware is calibrated at 20°C ($68^\circ\text{F}$). If your lab is a sweltering 30°C, your measurements are technically "wrong" before you even start the experiment.
The Role of Purity and Why "Tap" Isn't "True"
We need to address the elephant in the room: impurities. Most of us aren't using HPLC-grade, triple-distilled water. We’re using tap water. Tap water is a soup. It’s got minerals like calcium and magnesium, dissolved gases like oxygen and nitrogen, and sometimes a bit of chlorine or fluoride.
All those dissolved solids add mass without significantly changing the volume in the same proportion. This means tap water is almost always denser than pure water. In places with "hard water," like parts of the American Southwest or London, the density is noticeably higher than in places with "soft" water.
Salinity and the Ocean Factor
If you move from a kitchen to the coast, the rules of water density in mL change even more drastically. Seawater has an average density of about $1.025 \text{ g/mL}$. That sounds small, but it's the reason you float effortlessly in the Great Salt Lake or the Dead Sea. The salt adds massive amounts of weight (mass) to the water.
- Seawater contains roughly 35 grams of salt per liter.
- This increases the density because salt ions ($Na^+$ and $Cl^-$) tuck themselves into the spaces between water molecules.
- Cold, salty water is the "heaviest" water in the ocean, which drives the "Global Conveyor Belt"—the deep ocean currents that regulate our planet's climate.
Pressure: The Subtle Density Modifier
For most of us living at sea level, we think of water as "incompressible." And for 99% of applications, it is. But if you go deep enough—say, the bottom of the Mariana Trench—the sheer weight of the ocean above actually squeezes the water molecules closer together. Even though it's a liquid, the density increases by about 5% at the very bottom of the ocean. This isn't something you'll notice in a glass of water, but it's a massive deal for marine engineering and submersible design.
How to Measure Water Density at Home
If you're curious and have a decent digital scale, you can test this yourself. It’s a great way to see if your measuring tools are actually accurate.
- First, place a clean, dry measuring cup on your scale and "tare" it (set it to zero).
- Fill it to exactly the 100 mL line with room-temperature tap water.
- Check the weight. If it’s between 99g and 101g, you’re in the ballpark of standard water density in mL.
- Now, try it with hot water from the kettle (be careful!). You'll likely see a lower number.
- Try it with heavily salted water. The number will jump up.
This experiment proves why professional bakers prefer mass (grams) over volume (mL/cups). Volume is a liar. It changes with temperature, the shape of the container, and even how you pour. Mass is honest. A gram is a gram whether the water is boiling or freezing.
Common Misconceptions About the "1:1" Ratio
People often assume that because 1 mL = 1 gram for water, it works for everything. It doesn't.
A milliliter of honey weighs about 1.4 grams.
A milliliter of rubbing alcohol weighs about 0.79 grams.
Even in the world of milk, which is mostly water, the fat content changes the density. Whole milk is denser than skim milk because the fats and proteins add weight.
💡 You might also like: Now I Am Become Sleepy the Goer to Bed: Why This Meme Keeps Coming Back
Actionable Insights for Daily Life
Understanding water density in mL isn't just for people in white lab coats. It has real-world implications for how you interact with the world around you.
Precision Cooking and Baking
Stop using measuring cups for liquids if you want consistent results. Use a digital scale. Set it to grams and pour your water until the number matches the milliliters required. Because $1\text{ mL} \approx 1\text{ g}$, it makes the conversion effortless and significantly more accurate than squinting at a plastic line on a cup.
Aquarium Management
If you keep fish, especially saltwater fish, you probably use a hydrometer or refractometer. These tools are literally just measuring the density of your tank water. If the water evaporates, the salt stays behind, the density goes up, and your fish get stressed. Monitoring density is the only way to keep that ecosystem stable.
Home Maintenance
Ever wonder why your water heater has a "pressure relief valve"? It’s because as the water heater warms up, the water expands (density decreases, volume increases). Without a place for that extra volume to go, your pipes would literally explode.
👉 See also: Takumi Bistro & Bar: Why This Japanese Fusion Spot Actually Lives Up to the Hype
Scientific Literacy
Next time you see a "life hack" that claims you can identify "fake" honey or oil by how it sits in water, remember density. Most of these hacks are just basic physics. If a substance is denser than $1.0\text{ g/mL}$, it sinks. If it's less dense, it floats. It's not magic; it's just the molecules doing their thing.
To get the most accurate results in any project involving liquids, always calibrate your measurements to a temperature of 4°C if you need the "perfect" 1:1 ratio, or simply use a scale and accept a 0.3% margin of error at room temperature. For 99% of human endeavors, that tiny difference won't ruin your cake, but knowing it exists makes you a much sharper observer of the physical world.