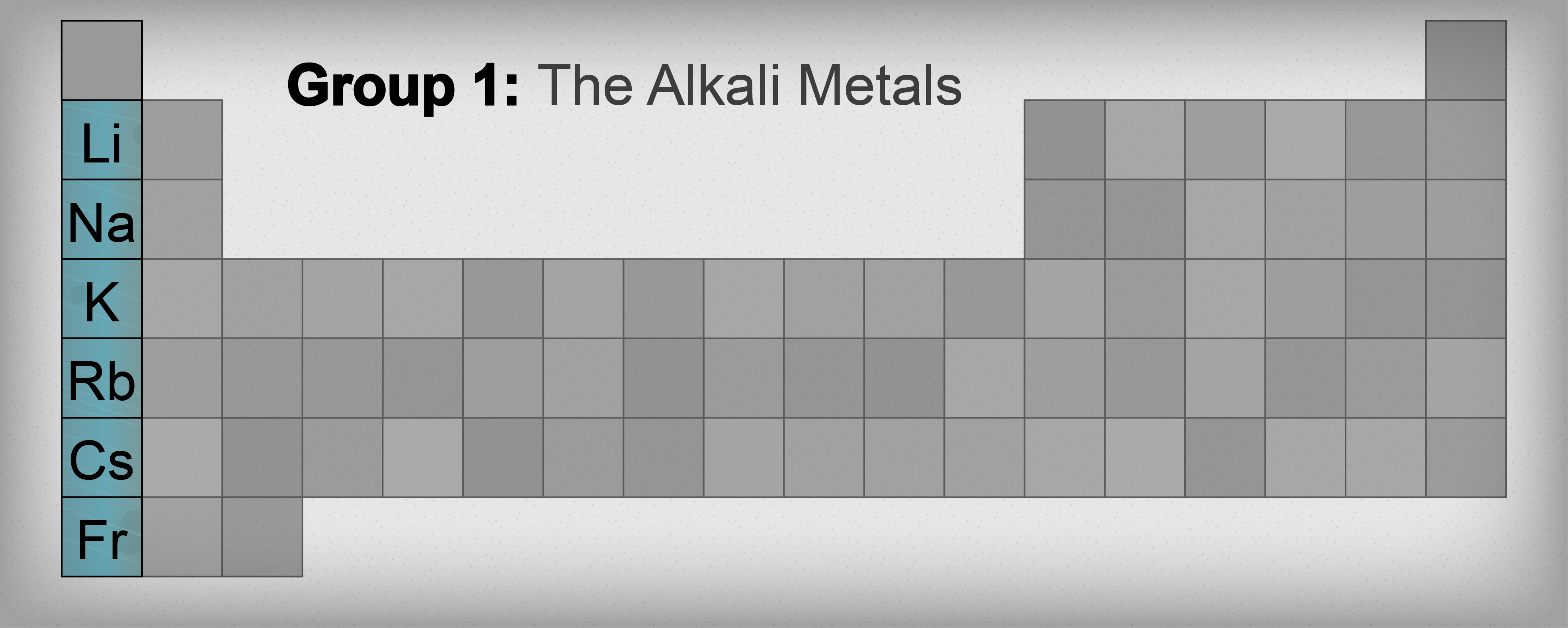
You're looking at a periodic table. It’s a mess of colors and letters. You need to find one specific family, the ones that explode in water and make life—literally—possible. So, what group are the alkali metals in? They are in Group 1. Right there on the far left. The very first column.
But there is a catch.
Most people see that column and assume everything in it is an alkali metal. It isn't. Hydrogen sits at the top like a squatter in a house it doesn't own. It's in Group 1, sure, but it’s a gas. It’s not a metal. The real alkali metals start with Lithium and go all the way down to Francium.
These elements are the rebels of the chemistry world. They are soft. You can cut Lithium with a butter knife. Try doing that with a piece of iron and you’ll just break your kitchenware. They are also incredibly "clingy" on an atomic level. They have one lone electron in their outer shell, and they want to get rid of it more than anything else in the world.
The Group 1 Identity Crisis
If you're asking what group are the alkali metals in, you're likely looking at the vertical columns of the periodic table. Scientists call these groups. Group 1 is home to Lithium (Li), Sodium (Na), Potassium (K), Rubidium (Rb), Cesium (Cs), and Francium (Fr).
Hydrogen is the oddball. It’s the "plus one" that wasn't actually invited to the party but showed up because it has one electron, just like the others. In reality, Hydrogen behaves more like a non-metal. It stays a gas under normal conditions, while its neighbors below are shiny, silvery solids.
Why does this group placement matter so much? Because chemistry is all about electrons. Every element in Group 1—excluding our friend Hydrogen—has a single valence electron. Think of this electron like a loose thread on a sweater. The atom wants to pull it off so it can reach a stable, "happy" state. This desperation to lose an electron makes these metals incredibly reactive.
You’ll never find a chunk of pure Sodium just sitting in a field. If it were there, it would have already reacted with the moisture in the air or the soil. In nature, these metals are always "married" to something else, forming compounds like Sodium Chloride (good old table salt).
🔗 Read more: Why the Pen and Paper Emoji is Actually the Most Important Tool in Your Digital Toolbox
Why Group 1 Metals Hate Water
If you’ve ever seen a high school chemistry teacher drop a tiny pea-sized bit of Sodium into a beaker of water, you know what happens next. It fizzes. It skims across the surface like a manic water strider. Sometimes it catches fire with a bright orange flame.
As you move down Group 1, things get scarier.
Potassium produces a lilac-colored flame. Rubidium? It basically explodes on contact. Cesium is even more violent. By the time you get to Francium, well, Francium is so radioactive and rare that you’ll likely never see enough of it in one place to watch it blow up.
This reactivity is the defining trait of what group are the alkali metals in. As the atoms get larger—meaning as you move down the column—that single outer electron gets further and further away from the positive pull of the nucleus. It's like a long-distance relationship. The further away the electron is, the easier it is for the atom to let go. This is why Cesium is way more reactive than Lithium.
Breaking Down the Members of the Family
Let’s look at who actually lives in this neighborhood.
Lithium is the lightest. It's the superstar of the modern world. Your phone, your laptop, and that electric car idling at the stoplight all rely on Lithium-ion batteries. It’s light, it holds a charge well, and it’s the "mildest" of the group.
Next is Sodium. You eat this every day. Without Sodium ions, your nerves wouldn't be able to send signals. Your heart wouldn't beat. It’s essential for life, yet in its pure metal form, it’s a dangerous substance that must be stored under oil to keep it from reacting with air.
💡 You might also like: robinhood swe intern interview process: What Most People Get Wrong
Potassium is the one you find in bananas, though not in its metallic form. It’s crucial for fluid balance in the body. If your Potassium levels get out of whack, you're in for a bad time.
Then we get into the heavy hitters. Rubidium and Cesium. These are used in atomic clocks. The "second" as we define it is actually based on the vibrations of a Cesium atom. It is the most accurate way we have to measure time.
Finally, there’s Francium. It’s the rarest naturally occurring element on Earth. At any given time, there might be less than thirty grams of it in the entire Earth's crust. It’s highly radioactive and disappears almost as soon as it’s formed.
The Physical Quirks of Group 1
When we talk about metals, we usually think of hard, dense things like gold or lead. Alkali metals flip that script.
- They are soft. You can literally squish them. Lithium is the hardest of the bunch, but even then, it's about as firm as cold butter.
- They have low melting points. Cesium will actually melt in your hand if you hold it (though please, don't ever try that; it’s toxic and would likely explode from the moisture on your skin).
- They are light. Lithium, Sodium, and Potassium are all less dense than water. If they didn't explode when they touched it, they would actually float.
These physical properties are all linked back to the same thing: their electron structure. Because they only have one electron to share in their "metallic bond," the glue holding the atoms together is pretty weak compared to something like Tungsten or Platinum.
Real World Applications: Not Just Explosions
It’s easy to focus on the "Group 1 go boom" aspect, but these elements are the backbone of modern tech.
Beyond batteries, Lithium is used in glass and ceramics to help them withstand heat. It’s also a powerful medication for bipolar disorder. Sodium vapor lamps provide that distinct yellow glow in streetlights.
📖 Related: Why Everyone Is Looking for an AI Photo Editor Freedaily Download Right Now
Potassium is a massive part of the global fertilizer industry. Without it, we couldn't grow enough food to feed eight billion people. It’s the "K" in the N-P-K label you see on bags of potting soil at the hardware store.
In the world of high-tech research, Rubidium is used to create Bose-Einstein condensates. That’s a state of matter where atoms get so cold they start acting like a single "super-atom." It’s weird quantum physics stuff that would be impossible without the specific properties of Group 1.
Common Misconceptions About Group 1
People often get confused when they see the periodic table because different versions use different numbering systems. You might see "Group 1A" in older textbooks. Don't let that trip you up. In the modern IUPAC system, it's just Group 1.
Another big mistake? Thinking that these metals are found "pure" in nature. They aren't. If you see a "vein" of Sodium in a rock in a movie, the writers didn't do their homework. You find them in salts, minerals, and seawater. Extracting the pure metal requires a process called electrolysis—essentially using electricity to force the metal away from its chemical partner.
Actionable Steps for Students and Hobbyists
If you are studying for a chemistry test or just trying to understand the world better, here is how you should handle the Group 1 elements:
- Memorize the "Hydrogen Exception." Always remember that while Hydrogen is at the top of the column, it is not an alkali metal. This is a favorite trick question for teachers.
- Learn the Trend. Understand that reactivity increases as you go down the group. Lithium is the "safest," and Francium is the most "dangerous."
- Check the Storage. If you ever work in a lab with these, always ensure they are submerged in mineral oil or an inert gas like Argon. Exposure to oxygen will tarnish them instantly, and exposure to moisture can be catastrophic.
- Connect to Life. Remember that Na (Sodium) and K (Potassium) are electrolytes. When you drink a sports drink after a workout, you're literally consuming Group 1 ions to keep your nervous system running.
The next time someone asks you what group are the alkali metals in, you won't just say "Group 1." You'll know they are the soft, reactive, electron-hating foundation of everything from your smartphone to your own heartbeat.