Think about your thumb for a second. If you look closely, you see skin patterns and maybe a tiny hangnail. But if you could zoom in—past the cells, past the proteins—you'd find atoms. Trillions of them. But here is the thing: the size of an atom is so ridiculously small that our brains aren't even wired to understand it properly. We’ve all seen the drawings in school textbooks. You know the ones. A solid ball in the middle with little planets orbiting around it. Honestly? That's a total lie.
Atoms are mostly empty space. Like, 99.99999% nothing. If an atom were the size of a football stadium, the nucleus would be a small marble sitting on the 50-yard line, and the electrons would be like tiny gnats buzzing around the very top rows of the stands. Everything in between? Empty. This realization is what makes the actual physical scale of our universe so hauntingly beautiful.
How Small Is the Size of an Atom, Really?
We need units that sound like science fiction to even talk about this. Scientists use the angstrom ($10^{-10}$ meters) or the picometer. For context, a single copper atom is roughly 128 picometers across. If you lined up 100 million of them, they would barely span the length of your fingernail. It's tiny.
Most people assume that because atoms make up "solid" things, they must be solid themselves. They aren't. When you "touch" a table, you aren't actually touching the atoms. You are feeling the electromagnetic repulsion between the electrons in your hand and the electrons in the table. You're basically levitating on a cushion of force. The size of an atom isn't a hard boundary like a marble; it's more like a fuzzy cloud of probability.
The Nucleus vs. The Electron Cloud
While the "size" of the whole atom is determined by where the electrons like to hang out, the nucleus is where all the meat is. The nucleus is about 100,000 times smaller than the atom itself. Yet, it contains more than 99.9% of the mass. This is where we get into the weirdness of density. If you had a sugar cube made entirely of atomic nuclei, it would weigh about 5 billion tons. That’s the weight of the entire human population many times over, packed into a dice.
Ernest Rutherford discovered this back in 1911 with his famous gold foil experiment. He shot alpha particles at a thin sheet of gold. He expected them to pass through like bullets through paper. Instead, some bounced straight back. He said it was as if you fired a 15-inch shell at a piece of tissue paper and it came back and hit you. This proved that the size of an atom is mostly a vast void with a tiny, dense core.
✨ Don't miss: Why Apple 29th Street Boulder Co Is Kinda the Best Place to Handle Your Tech Stress
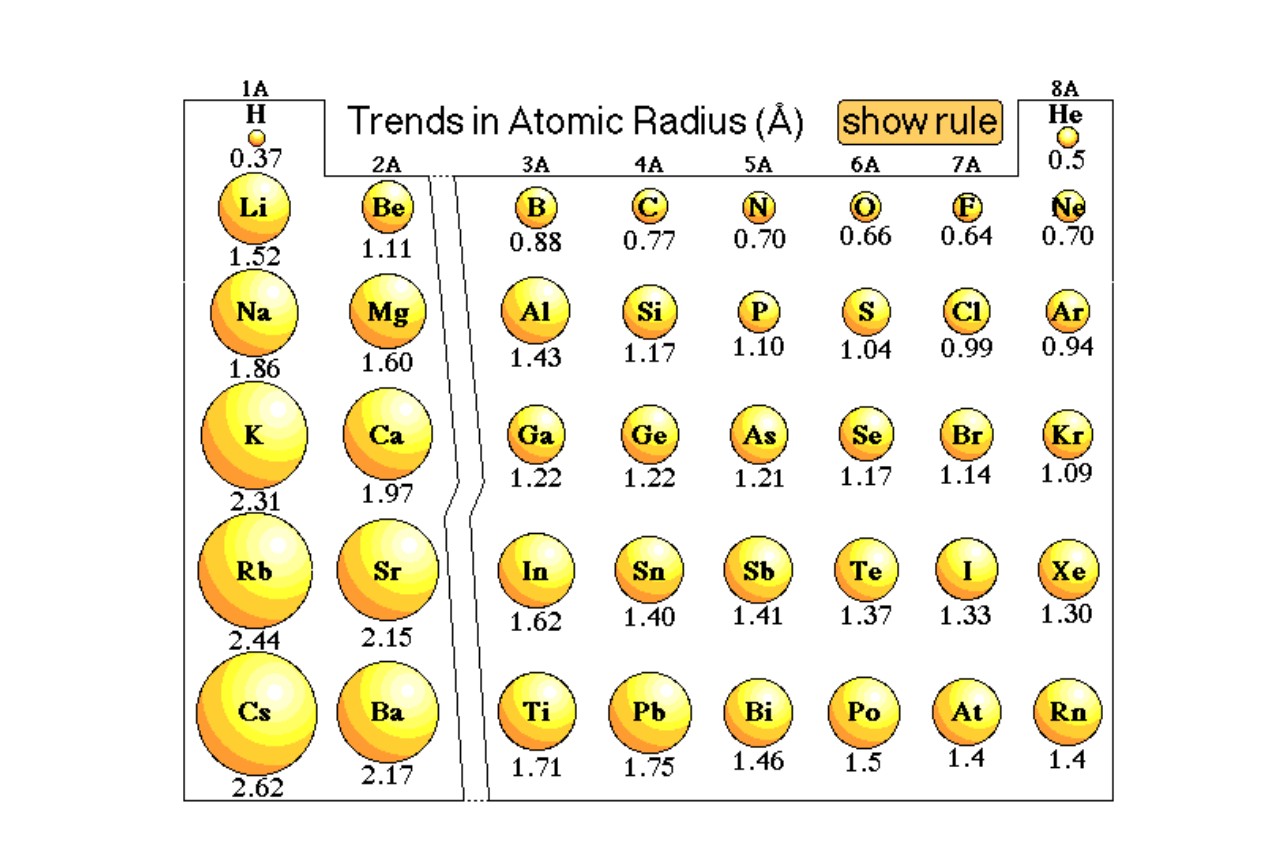
Why Do Different Atoms Have Different Sizes?
You might think that as you add more protons and electrons—going from Hydrogen to something heavy like Lead—the atom just keeps getting bigger and bigger in a linear way. But chemistry is weirder than that.
As you move across a row on the periodic table, atoms actually get smaller. This feels wrong, right? You're adding stuff, so it should grow. But because you're adding protons to the nucleus, the positive charge increases. This stronger "magnet" pulls the electron clouds in tighter. So, a Neon atom is actually smaller than a Lithium atom, even though it’s "heavier."
Then you have the "Shielding Effect." This is basically when inner electrons act like a screen, blocking the outer electrons from the pull of the nucleus. When you move down a column on the periodic table, you're adding entire new layers (shells) of electrons. This makes the size of an atom jump up significantly. Cesium is a absolute unit compared to Helium.
The Measurement Problem
Measuring these things isn't like using a ruler. Since atoms don't have a sharp edge, we use different "radii" depending on the situation:
- Atomic Radius: Usually half the distance between the nuclei of two identical atoms touching each other.
- Ionic Radius: The size after an atom loses or gains an electron (becoming an ion).
- Van der Waals Radius: The distance between atoms that aren't chemically bonded but are just chilling next to each other.
The Role of Quantum Mechanics
We can't talk about the size of an atom without mentioning Werner Heisenberg. His Uncertainty Principle basically says you can't know exactly where an electron is and how fast it's going at the same time. This is why we don't see "orbits." We see "orbitals"—calculated zones of probability.
✨ Don't miss: Verizon Network Status Page: What You Should Actually Check When Your Bars Vanish
The "size" of the atom is essentially the boundary where there is a 90% chance of finding an electron. If an electron gets excited by energy (like heat or light), it can jump to a higher "shell," momentarily increasing the atom's effective size. This is what happens when things expand when they get hot. The atoms aren't just vibrating more; their electron clouds are pushing further out.
Real-World Consequences of Atomic Scale
Why does any of this matter to you? Well, it’s the reason why your computer works. Transistors in modern microchips are now reaching sizes of 3 nanometers or 5 nanometers. That is only about 30 to 50 atoms wide. We are reaching the physical limit of how small we can make technology.
If we go any smaller, the size of an atom becomes a liability. Electrons can "teleport" through barriers because of a phenomenon called quantum tunneling. Basically, the walls of the transistor become so thin that the electron doesn't recognize them as a solid object anymore. It just blips to the other side, causing a short circuit.
- Scanning Tunneling Microscopes (STM): We can finally "see" atoms now. These microscopes use a needle that is only one atom wide at the tip. By measuring the electrical current between the tip and the surface, we can map out individual atoms.
- Nanotechnology: We are building machines at the scale of atoms. Imagine "molecular assemblers" that can build a diamond or a medicine molecule atom by atom.
- Material Science: The way atoms pack together—their size-to-space ratio—determines if a metal is brittle or if a diamond is hard.
Moving Forward with Atomic Knowledge
Understanding the size of an atom changes how you look at the world. You realize that everything "solid" is essentially a ghost. You're mostly made of nothing, held together by invisible forces.
To dive deeper into this, your next step should be looking into the Standard Model of Physics. While atoms are small, they are made of quarks and gluons which are even smaller—so small that we currently believe they have no physical size at all; they are "point particles."
You should also check out the work of Richard Feynman, specifically his "Plenty of Room at the Bottom" lecture. It’s the foundational text for nanotechnology and explains how much power we gain when we learn to manipulate things at the atomic scale.
✨ Don't miss: Google Maps Street View: What Everyone Gets Wrong About Privacy and Updates
Start by exploring the Interactive Scale of the Universe tools online. They allow you to scroll from the size of a galaxy all the way down to a Planck length. Seeing where the atom sits in that spectrum is a legitimate ego-check. It puts our entire existence into a very specific, very tiny perspective.