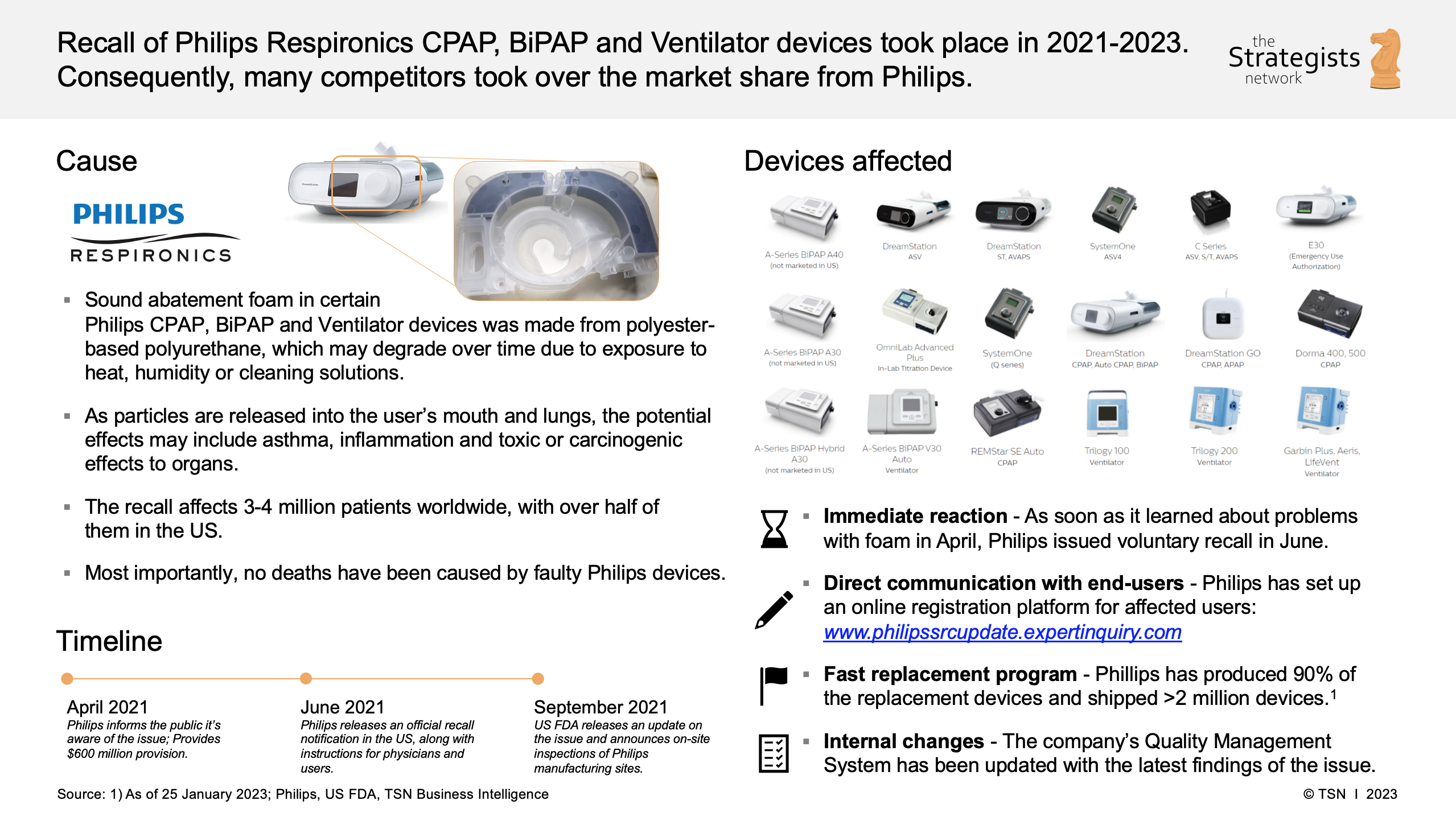
If you’re staring at that sleek, black CPAP machine on your nightstand and wondering if it’s secretly trying to kill you, you aren't alone. It’s a mess. Honestly, the Philips Dreamstation 2 recall situation is one of the most frustrating chapters in modern medical device history because it followed right on the heels of a much larger disaster.
You probably remember the 2021 chaos. Philips recalled millions of first-generation DreamStation machines because the sound-abatement foam—a polyester-based polyurethane (PEPUR) material—was literally disintegrating. People were inhaling tiny black flecks of foam and off-gassed chemicals while they slept. So, when the DreamStation 2 arrived, it was supposed to be the "safe" successor.
It wasn't that simple.
The DreamStation 2 uses a different type of foam (silicone-based), but new problems started cropping up. By late 2023 and into 2024, the FDA began issuing warnings about overheating, smoke, and even fire. This isn't just about bad foam anymore. It's about electrical components and design flaws that have left thousands of sleep apnea patients feeling totally abandoned by the brand they trusted to help them breathe.
Why the DreamStation 2 started smoking
It sounds like a bad joke. You get a replacement machine because the old one might give you respiratory issues, and the new one might set your house on fire. The FDA issued a safety communication specifically for the DreamStation 2 after receiving over 270 reports of thermal issues. We aren't just talking about a warm machine. We’re talking about reports of "fire, smoke, burns, and other signs of overheating."
The technical breakdown is a bit murky, but it basically boils down to electrical malfunctions. Unlike the original recall, which was a material science failure (the foam breaking down), the issues with the Philips Dreamstation 2 recall and safety alerts are rooted in the internal circuitry and the humidifier heater plate.
Imagine this: You're asleep, and the machine starts to smolder. According to the FDA, these incidents often happen when the machine is used with a humidifier. If the electrical components fail, they can overheat to the point of melting the plastic casing. It’s terrifying because these machines are literally strapped to your face. You are breathing in whatever fumes that melting plastic is producing.
💡 You might also like: How Much Should a 5'6 Female Weigh: What Most People Get Wrong
The silicone foam controversy
While the fire risk is the "new" headline, we have to talk about the foam again. Philips switched to silicone foam in the DreamStation 2 to avoid the breakdown issues of the PEPUR foam. However, the FDA hasn't been entirely happy with this switch either.
Testing on the silicone foam showed that while it doesn't break down the same way, it can still release certain chemicals—specifically volatile organic compounds (VOCs)—during the initial "off-gassing" period. Philips claims the levels are safe. The FDA, meanwhile, has been more cautious, noting that some testing didn't meet their rigorous standards for long-term safety.
It’s a classic case of corporate "good enough" versus regulatory "prove it." For the average person just trying to treat their sleep apnea, this back-and-forth is exhausting. Do you trust the manufacturer who already messed up once, or do you wait for a government agency that moves at the speed of a snail?
The 2024 Consent Decree: Philips stops selling
Things got real in early 2024. Philips reached a massive "consent decree" with the U.S. Department of Justice and the FDA. This is a big deal. Basically, it means Philips is legally prohibited from selling any new CPAP or BiPAP machines in the United States until they meet very specific, very difficult safety requirements.
This includes the DreamStation 2.
While the machine wasn't "recalled" in the sense that everyone had to send them back immediately (like the first one), Philips was essentially forced to stop its commercial operations for sleep therapy in the U.S. market. They are currently focused almost entirely on servicing existing machines and trying to fix their manufacturing processes.
What does this mean for you? If you have a DreamStation 2, you're likely stuck with it for now. Philips isn't sending out a different model because they don't really have one they’re allowed to sell right now.
Spotting the warning signs of a failing machine
You need to be your own quality control agent. If you’re using a DreamStation 2, don't just set it and forget it. You have to watch for very specific red flags.
- The Smell Test: If your machine smells like "hot electronics" or ozone, stop using it. Immediately. That is the smell of a circuit board or plastic getting way too hot.
- Visual Inspection: Look at the bottom of the machine and the humidifier area. Do you see any warping? Any discoloration of the plastic?
- Performance Glitches: If the machine starts restarting itself or the screen flickers, that’s an electrical fault. Don't wait for it to start smoking.
- Water Issues: Keep a close eye on the humidifier tank. If the water is disappearing faster than usual or if the plate at the bottom looks charred, unplug it.
Honestly, the safest bet for many has been to switch brands entirely. ResMed and Fisher & Paykel have seen a massive surge in users because people are just... done. But machines are expensive, and insurance companies are notorious for being difficult about "early replacements."
The legal fallout and your rights
There are massive class-action lawsuits happening right now. In mid-2024, a settlement was reached for economic loss—meaning people who bought the machines could get some money back. But that doesn't cover personal injury. If you actually got sick or were injured by a machine, those legal battles are still raging.
The Philips Dreamstation 2 recall and safety notices have made the legal landscape incredibly complex. Lawyers are looking at whether Philips knew about the overheating risks before they started shipping these as replacements for the original recalled units.
If your machine has failed, document everything. Take photos. Keep the unit (don't send it back to Philips without talking to a lawyer if you intend to sue).
How to move forward safely
The reality is that sleep apnea is dangerous. You can't just stop therapy because the machine is suspect. Untreated sleep apnea leads to heart disease, stroke, and extreme fatigue.
If you are using a DreamStation 2 and you're worried:
- Talk to your doctor first. Don't just stop using it. Ask them if they can advocate with your insurance for a "medical necessity" replacement for a different brand, like a ResMed AirSense 11.
- Register your device. Make sure your serial number is registered on the Philips website so you actually get the official notices.
- Use it on a hard surface. Never put your CPAP on a rug, a towel, or a bedspread. These machines need airflow underneath to stay cool. This is a common mistake that increases fire risk.
- Check your filters. A clogged filter makes the motor work harder. A harder-working motor gets hotter. Change them every two weeks, regardless of what the manual says.
It’s a tough spot to be in. You’re caught between a rock (respiratory issues) and a hard place (fire risk). But staying informed is the only way to navigate this. Philips has a long way to go to regain public trust, and for many, the DreamStation 2 was the final straw.
Immediate Actions to Take
Check your machine's serial number against the latest FDA safety communications. If you notice any "scorching" or "burning" smells, unplug the device immediately and contact both your DME (Durable Medical Equipment) provider and your physician. You should also file a report through the FDA’s MedWatch program; this is the only way the government tracks how widespread these failures actually are. If your insurance denies a replacement for a non-Philips brand, ask your doctor to file a formal "Letter of Medical Necessity" citing the FDA's warnings on thermal incidents.