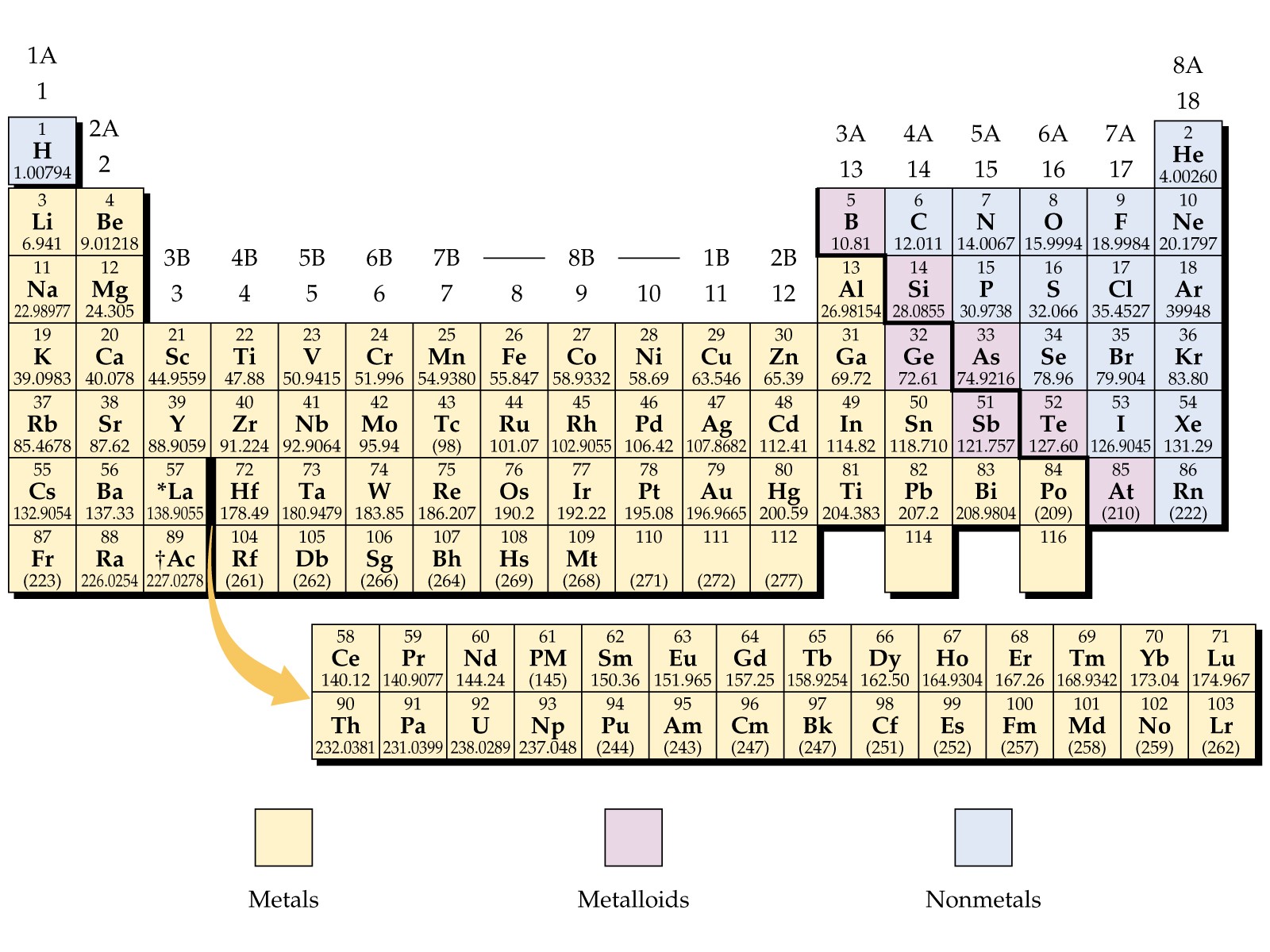
You probably remember it from high school. That weird, stepped "staircase" line zigzagging down the right side of the chart in your chemistry textbook. It looks like a mistake, honestly. But that dividing line on the metal non metal periodic table is basically the architectural blueprint for every piece of tech you’re currently using. Without that specific split, your smartphone is a brick, and your car is just a heap of useless ore.
Chemistry is messy. We like to pretend it’s all neat boxes and perfect numbers, but the reality of how elements behave is way more fluid. Most of the table—about 80%—is made of metals. They’re the heavy hitters, the shiny stuff that conducts heat and electricity like a pro. Then you’ve got the non-metals tucked away in the upper right corner, along with Hydrogen, which is basically the eccentric cousin living in the attic. In between? The metalloids. They’re the "confused" elements that can’t decide if they want to be a wire or an insulator.
The Physicality of the Metal Non Metal Periodic Table
Metals are the reliable workhorses. Think of Gold, Iron, or Copper. They share a "sea of electrons," which sounds like a bad sci-fi trope but is actually why they’re so good at what they do. Because their outer electrons are loosely held, they can flow. That’s electricity. They’re also malleable. You can smack a piece of Lead with a hammer, and it just flattens out. It doesn't shatter.
Try doing that with Sulfur.
Sulfur is a non-metal. It’s brittle. It’s yellow. If you hit it with a hammer, it turns into dust. Non-metals are the grumpy isolationists of the metal non metal periodic table. They hold onto their electrons with a death grip. This makes them terrible at conducting electricity, which, funnily enough, makes them perfect insulators. We need things that don’t move energy just as much as we need things that do.
The transition between these two isn't a hard wall. It’s a gradient. As you move from left to right across a period, elements get less metallic. They stop wanting to give away electrons and start wanting to hoard them. This tug-of-war is what creates chemical bonds. It's the reason we have water ($H_2O$) and salt ($NaCl$).
The Metalloid "No Man's Land"
Right on that staircase line sit the metalloids: Boron, Silicon, Germanium, Arsenic, Antimony, and Tellurium. These are the "maybe" elements.
Silicon is the king here. It looks metallic—it’s got that grey, shiny luster—but it’s brittle like a non-metal. More importantly, it’s a semiconductor. It only conducts electricity under certain conditions. This "sometimes" behavior is the entire basis of modern computing. By "doping" silicon with other elements from the metal non metal periodic table, we can create logical gates. 0s and 1s. Basically, every TikTok you watch is powered by the indecisive nature of a metalloid.
Breaking Down the Properties
If we’re being real, the "rules" for metals and non-metals are more like strong suggestions.
✨ Don't miss: How to Enable Apple CarPlay: Why Your Connection Keeps Failing and How to Fix It
- Luster: Metals are shiny because those free-flowing electrons reflect light. Non-metals are usually dull. Except for Diamond (Carbon), which is a non-metal but happens to be the sparkliest thing on Earth.
- State of Matter: At room temperature, almost every metal is a solid. Mercury is the weirdo that stays liquid. Non-metals? They’re all over the place. Oxygen is a gas, Bromine is a liquid, and Carbon is a solid.
- Density: Metals are generally dense. They’re heavy. Non-metals are the lightweights of the universe.
It’s easy to think of metals as "strong" and non-metals as "weak," but that’s a total myth. Carbon fibers are non-metals, and they’re stronger than steel. Graphene, a single layer of carbon atoms, is one of the toughest materials ever measured. It's all about how the atoms talk to each other.
The Real-World Friction
Where the metal non metal periodic table gets really interesting is in the "Post-Transition" section. Elements like Aluminum, Tin, and Lead. They are definitely metals, but they’re "soft." They have lower melting points than the transition metals like Tungsten or Platinum.
Then you have the Halogens and Noble Gases. These are the extreme non-metals. The Halogens (Fluorine, Chlorine) are chemically aggressive. They want electrons so badly they’ll rip them off almost anything else. On the flip side, the Noble Gases (Neon, Argon) are the introverts. They have a full set of electrons and don't want to talk to anyone. They’re the "perfect" non-metals because they’re completely non-reactive under normal conditions.
Why the Distinction is Fading in 2026
We used to treat the metal non metal periodic table as a rigid map. Today, materials science is blurring those lines. We’re creating metallic hydrogen under massive pressure—turning a gas into a metal. We’re making "organic metals" out of plastic polymers that conduct electricity.
The distinction still matters for basic chemistry, but in high-end tech, we’re looking at the behavior rather than the label. Researchers like those at MIT’s Department of Materials Science and Engineering are constantly pushing elements to act "out of character."
Take Boron. Usually, it’s a boring (pun intended) metalloid used in detergent. But engineer it correctly, and it becomes a high-strength component in aerospace armor. The periodic table isn't a static document; it’s a menu.
Sorting the Confusion
People often get tripped up by Hydrogen. It’s on the left side, which is "Metal Territory." But it’s a gas. Why? Because it has one electron, just like the Alkali metals (Lithium, Sodium). It fits there electronically, even if it doesn't fit physically. It’s the ultimate outlier.
Another common mistake is assuming all metals are magnetic. Nope. Iron, Nickel, and Cobalt are the famous ones. But Gold? Aluminum? Copper? They couldn't care less about your fridge magnets.
💡 You might also like: Call Apple Support for Apple ID: Why Most People Fail to Get Help
Actionable Insights for Using This Knowledge
If you’re a student, a hobbyist, or just someone trying to understand why your CPU gets hot, here is how you should actually look at the metal non metal periodic table:
- Check the Staircase: If an element is to the left of the line (excluding Hydrogen), expect it to be a conductor and a loser—of electrons, that is. It wants to form positive ions.
- Identify the "Bridge" Elements: If you’re working with electronics, focus on the metalloids. They are the interface between the digital and physical worlds.
- Look for Reactivity: The further you move toward the edges of the table (the far left or the far right), the more "desperate" and reactive the elements become. The middle (Transition Metals) is generally more stable.
- Ignore the Labels in Extremes: Under extreme heat or pressure, these categories fall apart. Don't rely on "metal" properties if you're designing something for a vacuum or a planetary core.
The metal non metal periodic table is a guide to the personality of matter. Metals are the socialites, sharing everything. Non-metals are the hoarders. Metalloids are the savvy negotiators. Understanding that dynamic makes the entire physical world a lot less mysterious and a lot more like a complex, ongoing conversation.