You’re looking at a mess of letters and numbers on a whiteboard. It looks like code. Honestly, it basically is. Chemistry is a language, and if you don't speak the dialect, you're just staring at alphabet soup. Symbols of chemical equations are the punctuation marks of the scientific world. Without them, we wouldn't know if a reaction is just sitting there or about to blow the windows out of the lab.
Most people think a chemical equation is just "A plus B equals C." It’s not. It’s a story. It tells you where things start, where they end, and exactly how they got there. If you miss a single small notation, like a tiny $(s)$ or a $(g)$, you might accidentally try to dissolve something that’s meant to be a solid chunk of metal. Chemistry doesn't forgive typos.
Why Symbols of Chemical Equations are the Secret Language of Matter
Think of a recipe. If it says "add eggs," you need to know if they should be room temperature, beaten, or boiled. In science, the symbols of chemical equations do that job. They provide the "how-to" for the universe.
The most basic one is the arrow $\rightarrow$. Everyone calls it the "yields" sign. It's the "then happens" part of the sentence. But did you know there are like, five different kinds of arrows? A single arrow means the reaction is a one-way street. You burn a piece of paper, you get ash. You aren't getting that paper back. But then you’ve got the double arrow $\rightleftharpoons$. That's the equilibrium sign. It means the chemicals are dancing back and forth, changing into each other and back again constantly. It’s a literal tug-of-war at the molecular level.
The States of Being
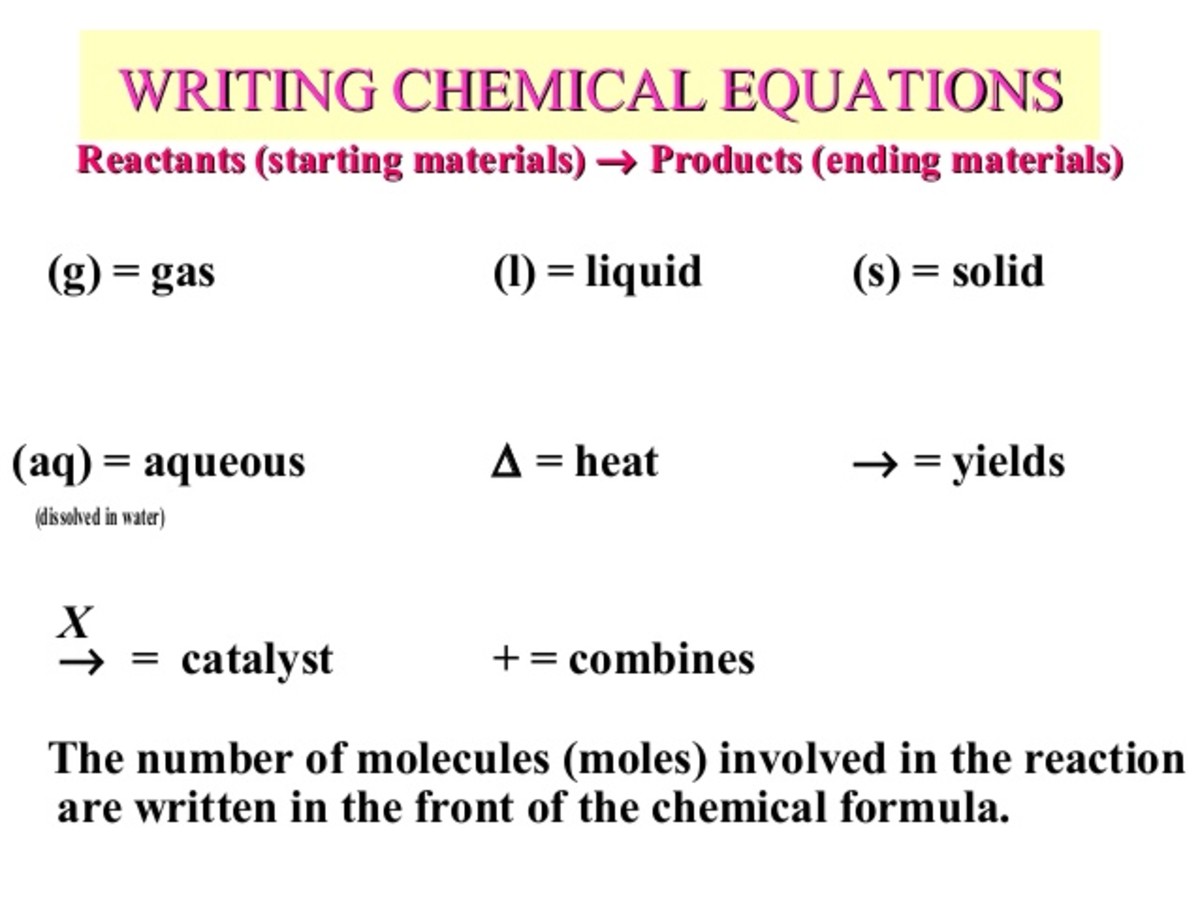
We’ve all seen the letters in parentheses. $(s)$, $(l)$, $(g)$, and $(aq)$. They seem simple, right? Solid, liquid, gas. But $(aq)$ is the one that trips everyone up. It stands for aqueous. Basically, it means the stuff is dissolved in water.
This matters more than you’d think. Salt as a solid $(s)$ is just something you put on fries. Salt as an aqueous solution $(aq)$ is a conductor of electricity. If you’re reading a safety data sheet or a lab manual, confusing $(l)$ for $(aq)$ can be the difference between a successful experiment and a literal fire. Pure liquid sodium is a terrifying, molten nightmare; sodium ions in water are just... salty water.
✨ Don't miss: iPhone 16 Pro Natural Titanium: What the Reviewers Missed About This Finish
The Weird Symbols You Might Have Missed
Sometimes you'll see a tiny Greek letter delta $\Delta$ sitting on top of the arrow. That’s not just for looks. It’s shorthand for "add heat." It’s like the "preheat oven to 350" instruction in a recipe. If you don't see that symbol, you might be waiting for a reaction to happen for a thousand years.
Then there’s the catalyst symbol. Usually, it’s a chemical formula like $Pt$ (Platinum) or $MnO_2$ written right above the arrow. The weird thing about catalysts? They don't actually get used up. They’re like the hammer in a construction project. The hammer helps build the house, but when the house is done, the hammer is still there, ready for the next job. In the world of symbols of chemical equations, the catalyst is the VIP that makes everything happen faster without getting its hands dirty.
Arrows Pointing Up and Down
In older textbooks, you might see an arrow pointing straight up $\uparrow$ or straight down $\downarrow$. They’re a bit old-school, but you’ll still find them in professional journals. An upward arrow means a gas was produced and bubbled out of the mixture. It’s escaping. A downward arrow means a "precipitate" formed. That’s a fancy way of saying a solid suddenly appeared out of a liquid and sank to the bottom like sand.
The Math Behind the Magic: Coefficients and Subscripts
Numbers are everywhere in these things. But they mean totally different things depending on where they sit.
- Subscripts: The tiny numbers like the $2$ in $H_2O$. These are non-negotiable. They tell you the "recipe" for the molecule itself. Change the subscript, and you change the substance. Change $H_2O$ to $H_2O_2$ and you’ve gone from life-giving water to hair bleach that will burn your throat.
- Coefficients: The big numbers in front, like $2H_2O$. These are just about quantity. It’s like saying "two bottles of water." You have more of the stuff, but the stuff itself hasn't changed.
Balancing these is the bane of every chemistry student’s existence. But honestly, it's just accounting. You can't just delete atoms because they're inconvenient. The Law of Conservation of Mass, famously championed by Antoine Lavoisier (the guy who basically lost his head during the French Revolution), says you have to end with exactly what you started with. If you start with four hydrogens, you better end with four hydrogens.
🔗 Read more: Heavy Aircraft Integrated Avionics: Why the Cockpit is Becoming a Giant Smartphone
Breaking Down a Real-World Example
Let's look at something you see every day: rusting iron. The equation looks like this:
$$4Fe(s) + 3O_2(g) \rightarrow 2Fe_2O_3(s)$$
Look at the symbols of chemical equations here. You have solid iron $(s)$ hanging out with oxygen gas $(g)$. They react to form iron(III) oxide, which is a solid $(s)$. The numbers tell us we need exactly four atoms of iron for every three molecules of oxygen. If the oxygen is too thin, the rust happens slower. If the iron is a liquid (which would be incredibly hot), the reaction would be violent. The symbols tell the whole story.
Pressure and Temperature Notations
In industrial chemistry—think about making fertilizer or plastic—the symbols get even more intense. You might see "$200 \text{ atm}$" or "$450^\circ\text{C}$" written over the arrow. This is the "Goldilocks" zone. If the pressure isn't exactly $200$ atmospheres, the nitrogen and hydrogen might just sit there and ignore each other. The Haber-Bosch process, which literally keeps half the world’s population fed through synthetic fertilizer, relies entirely on following these specific symbols.
Common Misconceptions About Equation Symbols
People often think that the "+" sign means "plus" like in math. It sort of does, but it really means "and" or "reacts with." When you see $A + B$, it’s not a sum. It’s an encounter.
💡 You might also like: Astronauts Stuck in Space: What Really Happens When the Return Flight Gets Cancelled
Another big mistake? Thinking that every equation you see on the internet is balanced. It’s usually not. People get lazy. They write $H_2 + O_2 \rightarrow H_2O$. That's a lie. It's physically impossible. You can't just lose an oxygen atom into the void. The real version needs those coefficients: $2H_2 + O_2 \rightarrow 2H_2O$.
The Role of Photochemistry
Ever see $h
u$ over an arrow? That’s not a typo. It’s the symbol for light (specifically, a photon's energy). You see this in photosynthesis equations. It means the reaction is "light-activated." Without a sunbeam or a lamp, nothing happens. It’s the ultimate "on" switch for life on Earth.
How to Read an Equation Like a Pro
If you want to master symbols of chemical equations, you have to read them from left to right like a sentence.
- Start with the Reactants: What do you have in your hand?
- Check the State Symbols: Is it a powder? A gas? Is it in water?
- Look at the Conditions: Do you need to heat it? Add a catalyst? Shine a light on it?
- Identify the Products: What did you actually make?
- Verify the Balance: Did any atoms disappear? (Hint: They shouldn't have).
Real-World Application: Why This Matters for You
You might think you’ll never use this unless you’re a scientist. Wrong. If you’ve ever mixed cleaning supplies, you’re doing chemistry. If you see a bottle that says "Contains Sodium Hypochlorite" and another that says "Ammonia," and you don't understand the potential reaction symbols, you could literally create toxic chloramine gas.
Knowing the symbols of chemical equations helps you understand the world’s "Warning" labels. It helps you understand why your car’s catalytic converter is so expensive (it's full of those $Pt$ and $Pd$ catalyst symbols). It even helps you understand food labels and why "hydrogenated" oils are a thing.
Actionable Next Steps for Mastery
If you're trying to get better at this, don't just memorize a list. That's boring and it won't stick. Try these instead:
- Deconstruct a Label: Grab a bottle of household cleaner or a snack bag. Look for chemical names and try to write out their basic symbols.
- Practice Balancing: Use an online simulator like PhET (University of Colorado Boulder). It makes balancing coefficients feel more like a puzzle game and less like homework.
- Visualize the States: Next time you see steam rising from a pot, mentally tag it as $H_2O(g)$. When you pour salt into that water, think $NaCl(aq)$.
- Check the Arrows: When reading science news, look at the diagrams. See if they use the $\rightleftharpoons$ symbol. It usually means the process is much more complex and harder to control than a simple one-way reaction.
Chemistry isn't just a subject; it's the operating system of the universe. The symbols are the code. Once you can read the code, the world starts making a lot more sense. You stop seeing "stuff" and start seeing processes. You see the heat, the pressure, and the constant movement of atoms trying to find a stable place to land.