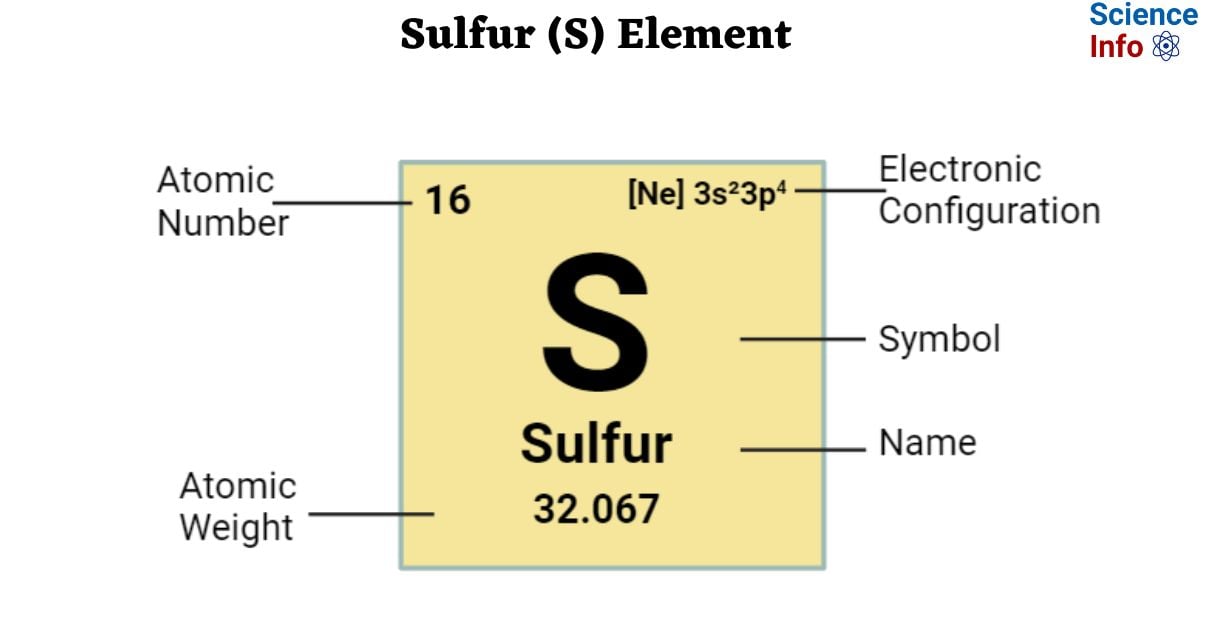
If you’ve ever cracked open a high school chemistry textbook, you’ve seen it. It sits there in Group 16, tucked between Oxygen and Selenium. It's the big, bold letter S. That is the symbol of sulphur. It’s arguably one of the easiest symbols to remember in the entire periodic table, unlike Lead (Pb) or Mercury (Hg), which seem designed to make students fail their exams.
But why just "S"? Why didn't it get stuck with something more complex? Honestly, the story of how we identify this "brimstone" element is a mix of ancient history, 19th-century logic, and some stubborn linguistic choices.
The Logic Behind the Symbol of Sulphur
The symbol of sulphur is S. It’s the 16th element. It has an atomic mass of about 32.06. While some elements take their symbols from Latin roots that sound nothing like their English names—think Ferrum for Iron—sulphur keeps it straightforward. The name itself comes from the Sanskrit word sulvere and the Latin sulfurium.
Back in 1813, a Swedish chemist named Jöns Jacob Berzelius decided the world of chemistry was a mess. People were using weird alchemical symbols—circles with arrows, crosses, and cryptic drawings—to represent elements. It was a nightmare for printing and even worse for teaching. Berzelius proposed that symbols should be letters. He suggested using the first letter of the Latin name of the element. Since the Latin name was Sulphur, we got the S. Simple. Effective.
Atomic Basics You Actually Need to Know
Sulphur isn't just a letter on a chart. It’s a non-metal. At room temperature, it’s a brittle, pale-yellow solid. If you’ve ever been to a volcanic hot spring or smelled a rotten egg, you’ve "encountered" sulphur.
$S_{8}$
👉 See also: Why Pictures That Shows Data Get Shared More Than Text Ever Will
The above formula represents the most common allotrope of sulphur. It doesn't just hang out as single atoms. It forms these neat little crown-shaped rings of eight atoms. This structure is what gives it that distinct yellow crystalline look. If you heat it up, those rings break and the whole thing turns into a dark, viscous goo that looks like something out of a horror movie.
Spelling Wars: Sulfur vs. Sulphur
You might notice I’m switching between "ph" and "f" here. It’s not a typo. It’s a geographical divide that has annoyed scientists for decades.
In the United States, the American Chemical Society (ACS) uses "Sulfur." It’s cleaner. It’s modern. In 1990, the International Union of Pure and Applied Chemistry (IUPAC)—the folks who basically act as the supreme court of chemical naming—decided that "Sulfur" would be the international standard.
However, the UK, India, and many other Commonwealth countries grew up with "Sulphur." Even though the "f" spelling is technically the official one for scientific papers, the "ph" version remains deeply embedded in the English language and history. No matter how you spell it, the symbol of sulphur remains S. It’s the one thing everyone agrees on.
Why the Symbol Matters in Industry
We don't just stare at the symbol S in labs. It’s everywhere. Honestly, the amount of sulphuric acid a country produces is often used as a shortcut to measure how well its economy is doing. It’s that important.
Most of the world's sulphur is a byproduct of oil and gas processing. When we "sweeten" natural gas, we're basically stripping the sulphur out so it doesn't turn into acid rain when the fuel is burned. That recovered sulphur is then turned into fertilizer. Without it, global food production would basically collapse.
- Vulcanization: This is the process that makes rubber durable. Without sulphur, your car tires would be a sticky mess in the summer and a brittle rock in the winter.
- Matches and Gunpowder: The "brimstone" of the Bible is sulphur. It’s been used in explosives and incendiary devices for thousands of years because it burns easily.
- Medicine: Sulfa drugs were the first chemical substances used systematically to treat bacterial infections in humans. They paved the way for the antibiotic revolution.
Surprising Places You’ll Find Sulphur
It’s in your hair. Specifically, in the keratin protein. The "S-S" bonds (disulphide bridges) are what give your hair its strength and shape. When someone gets a "perm," they are literally using chemicals to break and then reform the sulphur bonds in their hair to change its texture.
It’s also in garlic and onions. The reason your eyes water when you chop an onion is due to a volatile sulphur compound called syn-propanethial-S-oxide. When it hits the water in your eyes, it creates a tiny amount of sulphuric acid. Your eyes sting because they are literally being attacked by a mild acid.
Misconceptions About the Element
People think sulphur itself smells like rotten eggs. It doesn't. Pure, elemental sulphur is actually odorless. The "stink" we associate with it usually comes from Hydrogen Sulfide ($H_{2}S$). That’s the gas produced by bacteria breaking down organic matter in swamps or your gut.
👉 See also: Finding the Formula for X and Y Intercept Without Losing Your Mind
Another weird fact? In the deep ocean, near hydrothermal vents, there are entire ecosystems that don't rely on the sun. Instead of photosynthesis, they use chemosynthesis. They "eat" the sulphur compounds coming out of the earth's crust. It’s as close to alien life as we’ve found on Earth.
Practical Takeaways for Your Next Chemistry Test or Project
If you're trying to master the periodic table, don't overthink the symbol of sulphur. It's just S. But remember these nuances to show you actually know your stuff:
- Placement: It's right under Oxygen. They share similar outer electron shells, which is why sulphur can often stand in for oxygen in certain biological molecules (though the results are usually smellier).
- Valency: It’s versatile. It can have oxidation states ranging from -2 to +6. This flexibility is why it can be used in everything from battery electrolytes to skin creams for acne.
- The "f" vs "ph" rule: Use "Sulfur" for formal scientific reports if you want to follow IUPAC, but don't be surprised if your older textbooks or British friends stick with "Sulphur."
- Safety: While elemental sulphur is relatively low-toxicity, its compounds like $SO_{2}$ (sulphur dioxide) are major respiratory irritants. Always handle sulphur compounds in well-ventilated areas.
To really get a handle on this, try looking at the ingredients on your shampoo bottle or a bag of garden fertilizer. You’ll see "sulfates" or "sulfites" everywhere. Recognizing that "S" in the middle of those long chemical names helps you understand that this ancient "burning stone" is still doing the heavy lifting in our modern world.
For your next step, check out the Reactivity Series or look into how the Contact Process converts raw sulphur into sulphuric acid. Understanding the symbol is just the entry point to seeing how this element builds the world around us.