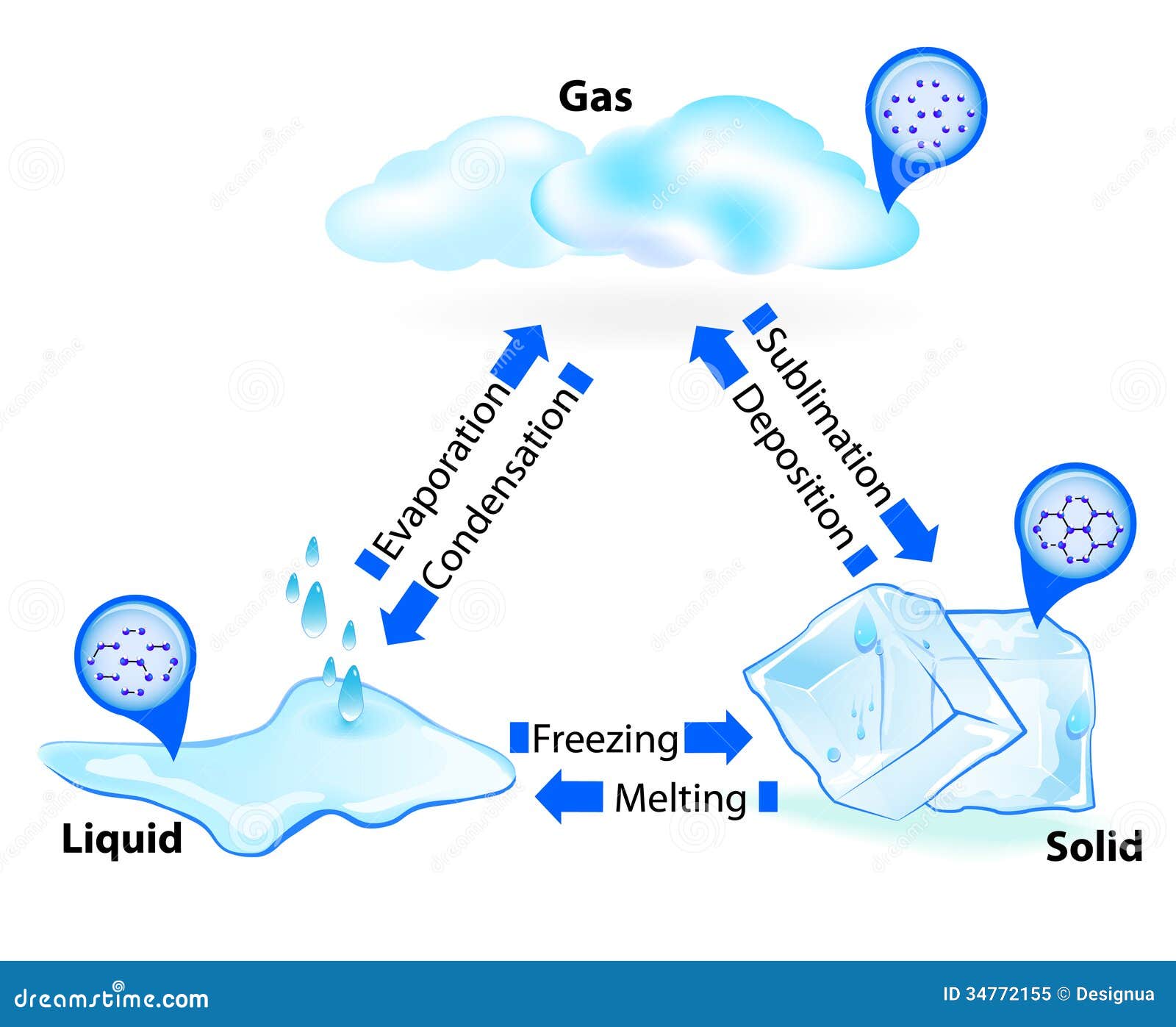
Look at any states of matter diagram in a middle school textbook. You’ll see three neat little boxes. One has balls packed tight like a crate of oranges. That’s your solid. The next has them rolling around like marbles in a jar—the liquid. The last one shows them flying everywhere like angry bees. That’s the gas. It’s clean. It’s simple. It’s also wildly incomplete.
We’ve been taught this "Big Three" model since we were five. But honestly, if you’re trying to understand how the universe actually holds itself together, that basic triangle doesn't cut it. It leaves out the fact that most of the visible universe isn't even in those three states. It ignores what happens when things get weirdly cold or terrifyingly hot.
Science isn't just a list of definitions. It's messy.
The hidden complexity behind your states of matter diagram
Most people think of phase changes as a toggle switch. You turn the stove on, the ice melts. Simple, right? But a truly accurate states of matter diagram needs to account for energy density and molecular arrangement in ways that a static image just can't capture.
Take water. We all know it as the "poster child" for phase changes. But water is a bit of a rebel. Most substances shrink when they freeze because the molecules stop dancing and huddle together for warmth. Water? It expands. It builds these lattice structures that take up more space, which is why your pipes burst in January. If you drew a states of matter diagram specifically for water, the "solid" section would actually look less dense than the "liquid" section.
Then you’ve got the transitions. We talk about melting and boiling, but what about sublimation? That's when a solid just gives up on being a liquid and jumps straight to gas. Think of dry ice. It’s carbon dioxide that’s so "impatient" it skips the puddle phase entirely. On the flip side, you have deposition, where gas turns directly into a solid, like frost forming on a cold windshield.
Why plasma is the "forgotten" fourth state
If you look at a states of matter diagram used by NASA or a plasma physicist, it looks nothing like the one in a 6th-grade classroom. Plasma is usually relegated to a footnote. That’s hilarious when you realize that plasma makes up about 99% of the visible matter in the universe.
✨ Don't miss: New DeWalt 20V Tools: What Most People Get Wrong
Stars. Lightning. Neon signs. The "northern lights." All plasma.
So what actually happens there? You take a gas and you keep pumping energy into it. Eventually, the electrons get so fed up with being tied to the nucleus that they just leave. They break free. Now you have a soup of ions and electrons. This soup conducts electricity. It reacts to magnetic fields. It’s why the sun has those massive solar flares that can knock out our satellites.
If you aren't including plasma in your mental states of matter diagram, you're basically ignoring the entire sky.
The weird stuff: BECs and Superfluids
Now, let's go the other way. What happens when you suck all the energy out?
In 1995, Eric Cornell and Carl Wieman cooled rubidium atoms to a temperature so low it’s hard to even wrap your head around—nearly absolute zero ($0K$ or $-273.15^\circ C$). At this point, the atoms lose their individual identity. They overlap. They behave like a single "super-atom." This is the Bose-Einstein Condensate (BEC).
In a BEC, the states of matter diagram basically breaks. You can’t point to one atom because they are all everywhere at once.
🔗 Read more: Memphis Doppler Weather Radar: Why Your App is Lying to You During Severe Storms
Then there are superfluids. Liquid helium, when cooled enough, becomes a superfluid. It has zero viscosity. If you put it in a cup, it will literally crawl up the sides and leak out over the top. It defies gravity because there's no friction to hold it back. It’s the kind of thing that makes you realize our everyday experience of "solid, liquid, gas" is just a tiny slice of reality.
The role of pressure (The variable we always forget)
Most people only think about temperature. Heat it up, it melts. Cool it down, it freezes. But pressure is the secret sauce.
If you go to the top of Mount Everest, you can't make a decent cup of tea. Why? Because the air pressure is so low that water boils at about $70^\circ C$ ($160^\circ F$). That’s not hot enough to properly extract the flavor from the leaves. In this environment, your states of matter diagram shifts. The "liquid" zone shrinks.
Down in the depths of the ocean, or inside a planet like Jupiter, pressure is so high that things get truly bizarre. We’re talking "metallic hydrogen." Under extreme pressure, hydrogen—a gas you use to fill balloons—turns into a metal that conducts electricity. This isn't just theory; researchers at places like the Lawrence Livermore National Laboratory use massive lasers to try and recreate these states.
Making the diagram work for you
If you're a student, a teacher, or just a curious human, don't settle for the triangle. A real states of matter diagram is more like a map of a landscape.
- The Valleys (Solids): Low energy, high stability. The atoms are vibrating, but they aren't going anywhere.
- The Plains (Liquids): Moderate energy. The atoms are touching, but they’re sliding. They take the shape of the container but keep their volume.
- The Atmosphere (Gases): High energy. Total chaos. They fill whatever space you give them.
- The Ionosphere (Plasma): Extreme energy. The atoms themselves break apart.
Practical implications of phase changes
This isn't just academic. Understanding these transitions is how we have modern life.
💡 You might also like: LG UltraGear OLED 27GX700A: The 480Hz Speed King That Actually Makes Sense
- Refrigeration: Your fridge works by forcing a substance to change from a liquid to a gas and back again. This process absorbs heat from inside the box and dumps it out the back. It’s literally a mechanical states of matter diagram in your kitchen.
- Lyophilization: That’s a fancy word for freeze-drying. By lowering the pressure, we can sublimate the water out of food. It preserves the structure and nutrition without cooking it.
- Aerosol Science: Understanding how liquids turn into fine mists (gases/suspensions) is how we design everything from asthma inhalers to fuel injectors in car engines.
Where the science is heading
We are finding new states all the time. Time crystals. Rydberg polaron. Quark-gluon plasma.
The last one is what existed microseconds after the Big Bang. It’s a "perfect fluid" where even protons and neutrons melt into their constituent parts. We recreate this at the Large Hadron Collider (LHC). It’s the hottest stuff ever made on Earth, trillions of degrees.
When you look at a states of matter diagram, remember it’s a snapshot. It’s a simplification of a universe that is constantly vibrating, shifting, and changing.
Actionable Insights for Deeper Learning
To truly master this concept, don't just stare at a picture. Try these steps:
- Experiment with non-Newtonian fluids: Mix cornstarch and water (Ooze). It behaves like a solid when you punch it and a liquid when you hold it. It’s a great way to see how the "standard" states have blurry edges.
- Study Phase Diagrams: Move past the simple "three boxes" and look up a pressure-temperature phase diagram for water or carbon dioxide. Look for the "triple point"—the exact temperature and pressure where a substance exists as a solid, liquid, and gas all at the same time.
- Observe Sublimation: Buy some dry ice (carefully!) and watch it skip the liquid phase. It's the most visual way to see that the "solid-to-liquid-to-gas" path isn't the only way to travel.
- Check out the NASA Cold Atom Lab: Look up the work being done on the International Space Station. They are creating BECs in microgravity because it allows them to observe these weird states for longer than we can on Earth.
The universe isn't a textbook. It's a high-energy dance floor. Your job is just to figure out which song is playing.