You’ve been there. You mix two clear liquids, expecting a beautiful, cloud-like precipitate to drift to the bottom of the beaker, but nothing happens. Or worse, you’re staring at a muddy mess when you expected a clear solution. It’s frustrating. Most of the time, the culprit isn't your technique—it’s that you didn't check the solubility table properly. Chemistry isn't just about mixing stuff; it’s about predicting the stubbornness of ions.
Solubility is a weirdly specific thing. Some salts, like Sodium Chloride, will dissolve in water until the cows come home. Others, like Silver Chloride, hit the water and basically turn into stone immediately. Understanding this isn't just for passing a midterm. It’s the foundation of wastewater treatment, pharmaceutical design, and even how your body processes minerals. If you don't know which ions hate each other, you're just guessing.
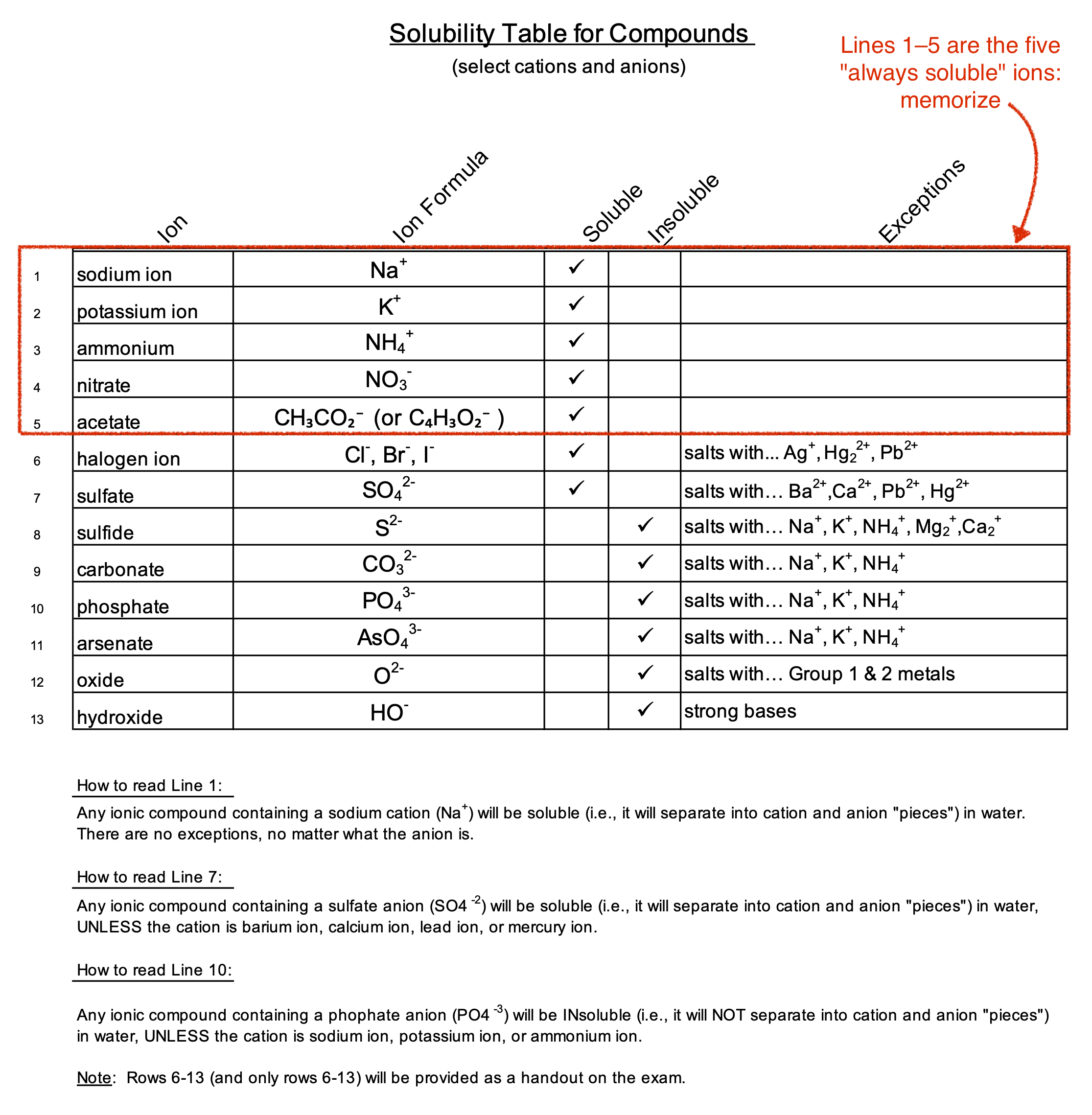
What is a Solubility Table Anyway?
Think of a solubility table as a social map for chemicals. It tells you who is going to hang out together in a liquid and who is going to "break up" and crash out as a solid. In technical terms, it’s a chart that lists the combinations of anions and cations and tells you if they are soluble (S), insoluble (I), or slightly soluble (s) in water at standard temperature.
Usually, these tables are organized by the negative ion—the anion. You look up your anion, find your cation, and see where they intersect. If you see an "I," you’ve got a precipitate. If it’s an "S," they stay dissolved. It sounds simple, but there are layers to this. Temperature changes everything. Pressure matters for gases. But for most lab work, the standard table is your bible.
The Rule-Breakers and the Reliables
There are some ions that just refuse to play hard to get. Group 1 elements? Lithium, Sodium, Potassium—they are the social butterflies of the periodic table. They are almost always soluble. No matter who they’re paired with, they stay in the solution. Nitrates ($NO_3^-$) and Acetates ($C_2H_3O_2^-$) are the same way. You can almost bet your life that if a compound has a nitrate in it, it’s going to dissolve.
Then you have the stubborn ones. Carbonates ($CO_3^{2-}$) and Phosphates ($PO_4^{3-}$) are generally the introverts. They don’t want to be in the water. They want to stick to their partner and sit at the bottom of the tank. Unless they are paired with a Group 1 metal, they are usually insoluble. This is why hard water is such a pain; the calcium and magnesium ions find carbonates and form that crusty "scale" in your tea kettle.
Why Does It Actually Matter?
It's easy to think this is just academic fluff. It’s not. If you are a doctor prescribing medication, solubility determines if the drug enters the bloodstream or just passes through the gut unused. In environmental engineering, we use the solubility table to pull toxic heavy metals out of industrial runoff. If you have lead in the water, you add something that makes it insoluble, like sulfate or sulfide, so you can literally filter the "poison" out as a solid.
Ever wonder why the ocean is salty but not "sandy" with dissolved minerals? It's a massive, ongoing chemistry experiment governed by these exact rules. The equilibrium between dissolved ions and solid deposits creates the very chemistry of our planet.
The Fine Print: Exceptions to the Rules
Chemistry loves to give you a rule and then immediately tell you why it’s wrong. Take Halides (Chlorides, Bromides, Iodides). Generally, they are very soluble. You put salt in water, it disappears. Easy. But then come the "Three Musketeers of Insolubility": Silver ($Ag^+$), Mercury ($Hg_2^{2+}$), and Lead ($Pb^{2+}$).
If you pair Silver with Chloride, it’s game over for the solution. You get a thick, white precipitate of $AgCl$. Interestingly, Lead(II) Chloride is a bit of a weirdo—it’s insoluble in cold water but starts to dissolve quite well if you heat the water up. This is why "slightly soluble" is such an annoying category on the solubility table. It’s a gray area.
Let's Talk About Thermodynamics
Why does one thing dissolve and another doesn't? It’s a fight between two forces: Lattice Energy and Hydration Energy.
- Lattice Energy: This is how badly the ions want to stay stuck together in a solid crystal.
- Hydration Energy: This is how badly the water molecules want to surround those ions and pull them away.
If the water’s "pull" is stronger than the crystal’s "hold," the substance dissolves. If the lattice is too strong—like in the case of many oxides—the water just bounces off, and the solid stays solid. This is why $Al_2O_3$ (Aluminum Oxide) doesn't dissolve in your soda can; the lattice energy is massive.
Common Misconceptions People Have
A huge mistake people make is thinking that "insoluble" means zero dissolution. That’s a lie. In reality, everything dissolves a little bit. Even a piece of gold in the ocean loses a few atoms to the water. In chemistry, we use something called $K_{sp}$ (the solubility product constant) to measure this.
When a solubility table says something is insoluble, it really means the amount that dissolves is so tiny it’s negligible for most purposes. But for high-stakes science, like nuclear waste storage or precision medicine, that "negligible" amount can be a huge deal.
Another weird one? People think adding more water always helps. While true for simple solubility, if you have a saturated solution at a specific temperature, you’re just making more volume of a solution that’s still at the same concentration limit.
How to Use This in Your Lab Today
Don't just memorize the table. It’s a waste of brain space. Instead, memorize the "Always Soluble" list and the "Big Exceptions."
- NAG SAG: This is an old mnemonic. Nitrates, Acetates, Group 1. Sulfates, Ammonium, Group 17.
- The Exceptions for Sulfates: $Ba^{2+}$, $Sr^{2+}$, $Pb^{2+}$.
- The Exceptions for Group 17: $Ag^+$, $Pb^{2+}$, $Hg_2^{2+}$.
If you know those, you can navigate 90% of a chemistry lab without glancing at a chart.
The Future of Solubility Research
We are getting better at "forcing" things to be soluble. This is huge in the pharmaceutical world. About 40% of new chemical entities discovered by drug companies are practically insoluble in water. That means they are useless as pills because the body can't absorb them. Scientists are now using "nanocrystals" and "amorphous solid dispersions" to trick these molecules into dissolving.
📖 Related: Free Software for Music: Why You Don't Need a Mortgage to Build a Studio
Basically, we are learning how to hack the solubility table. By messing with the physical structure of a substance, we can make an "insoluble" chemical behave like a soluble one just long enough to get it where it needs to go in the body.
Actionable Steps for Mastering Solubility
If you're struggling to make sense of your results, stop guessing. Follow these steps:
- Check the Cation First: If it’s Sodium, Potassium, or Ammonium, stop. It’s soluble. Move on.
- Identify the Anion: Look for Nitrates or Acetates. Again, if they’re there, it’s soluble.
- Watch for the Heavy Hitters: If you see Silver or Lead, be suspicious. They are the most common reasons for unexpected precipitates.
- Temperature Check: If your "insoluble" gunk isn't appearing, check your temperature. Many substances become much more soluble as the liquid gets hotter.
- Cross-Reference $K_{sp}$ Values: If you’re doing advanced work, the solubility table isn't enough. You need the actual $K_{sp}$ value from a handbook like the CRC Handbook of Chemistry and Physics. This will give you the mathematical limit of how much will dissolve.
Chemistry isn't magic; it’s just a set of rules about who wants to hold hands and who wants to let go. Once you get the hang of the table, you'll stop seeing "failed experiments" and start seeing the predictable dance of ions.
Next time you’re in the lab, grab a copy of the table, but try to predict the outcome before you look. That’s how you actually learn the logic behind the liquid.