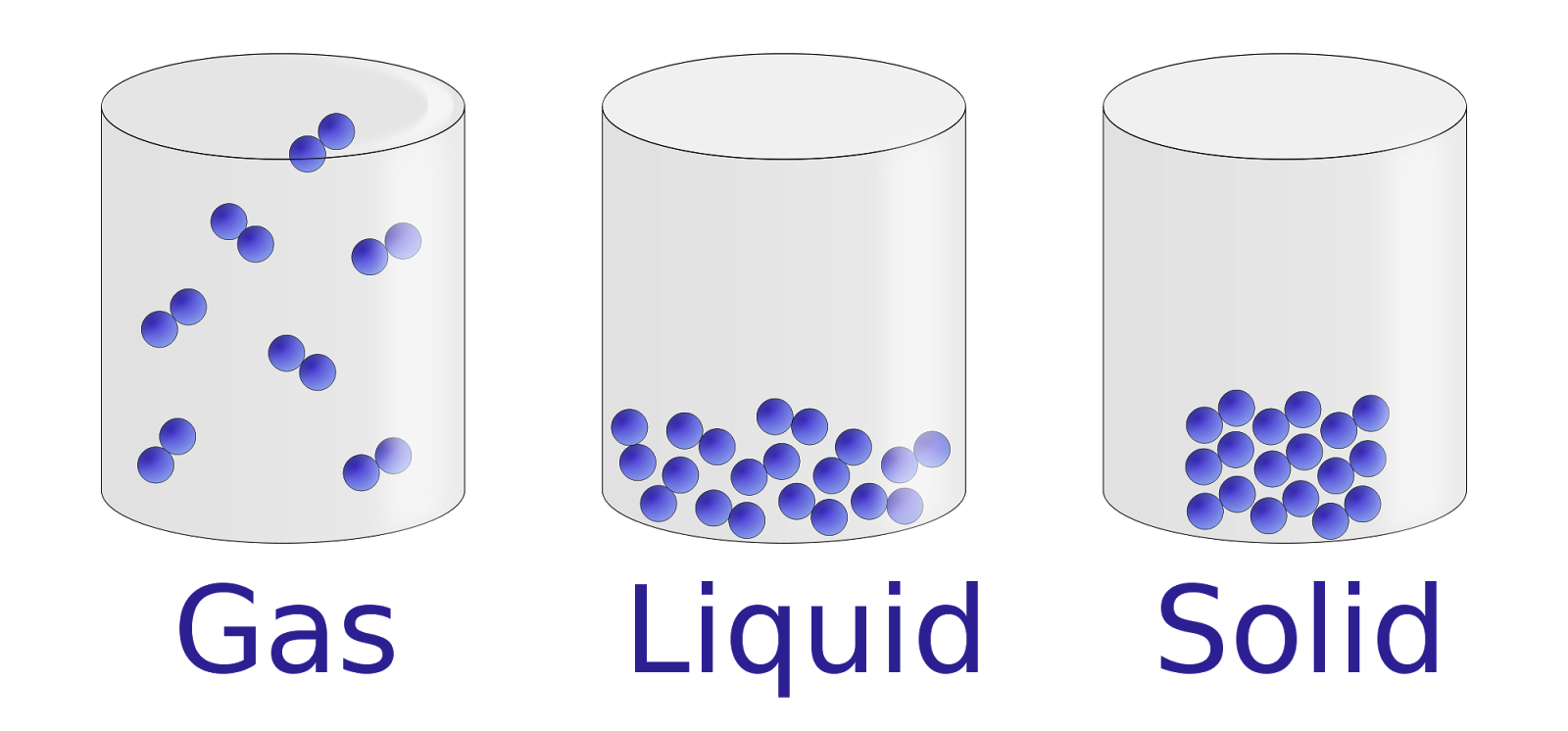
You've seen them a thousand times. Those little diagrams with the neat circles. In the "solid" box, they're all lined up like soldiers in a parade. In the "liquid" one, they’re a bit messy, like a ball pit at a birthday party. Then you hit the "gas" box, and it’s just three or four circles flying around with little "whoosh" lines behind them.
Honestly? These solid liquid gas images are mostly lies.
Well, not lies, exactly. They're simplifications. But if you’re trying to understand how the world actually works—or if you’re a student, a teacher, or just someone down a late-night Wikipedia rabbit hole—those basic drawings can actually make it harder to grasp the weirdness of physics. We live in a world where glass behaves like a liquid but looks like a solid, and where "supercritical fluids" exist that are basically both at the same time.
Understanding the visual representation of matter isn't just for passing a chemistry quiz. It’s how we design better phone screens, how we store rocket fuel, and how we understand the very air we’re breathing right now.
The Problem With the "Ball and Stick" Model
Most solid liquid gas images use what we call the kinetic molecular theory as a cheat sheet. It's a great starting point. It tells us that everything is made of particles and those particles are always moving.
But here’s the kicker: the scale is almost always wrong.
In a solid, like a diamond or a chunk of iron, those atoms are locked in a crystal lattice. But they aren't sitting still. They are vibrating like crazy. If you could actually see a "still" image of a solid at the atomic level, it would be a blur of motion. The images we see in textbooks make it look static. It’s not. It’s a mosh pit where nobody is allowed to move their feet.
Then you have liquids. Most images show liquid particles with huge gaps between them. In reality? Water molecules are almost as crowded as ice. The difference isn't the space; it’s the freedom. In a liquid, the particles are constantly sliding past each other, breaking and forming bonds in picoseconds. If you’re looking at solid liquid gas images that show liquids as "loose solids," you're getting a half-truth.
Why Gas Images Are the Biggest Offenders
Gases are the hardest to draw. Why? Because the empty space is the whole point.
If you drew a gas to scale based on a real-life room, your screen would be 99.9% empty. You’d have one tiny pixel in the top left and maybe another in the bottom right. To make it "look" like something, illustrators cram twenty "molecules" into a tiny box. This gives people the wrong idea about pressure and mean free path—the distance a particle travels before it smacks into something else.
In a real gas at room temperature, a molecule is traveling at hundreds of meters per second. It’s a chaotic, high-speed bumper car arena. Those cute little drawings with the three circles? They don't capture the violence of it.
When the Lines Get Blurry: Beyond the Basics
We love categories. We love neat boxes. But nature is messy.
Take glass. If you look at solid liquid gas images, glass looks like a solid. It’s hard. You can break it. It holds its shape. But look at the atoms. They aren't in a neat lattice like a metal or a salt crystal. They are disordered, frozen in a random mess. This is called an amorphous solid. Some scientists argue it’s actually more like a liquid that’s just moving so slowly it would take billions of years to notice.
Then there’s the fourth state: Plasma.
Most people forget plasma because it doesn't fit into the three-box graphic very well. But plasma is actually the most common state of matter in the universe. It’s what stars are made of. It’s what’s inside a neon sign. Visually, it looks like a gas, but the atoms have been stripped of their electrons. It’s electrically charged. It glows. It responds to magnets. If your collection of solid liquid gas images doesn't include a glowing, crackling purple box for plasma, it's incomplete.
The Weird World of Supercritical Fluids
Ever heard of decaf coffee? You can thank supercritical CO2 for that.
📖 Related: Why Please Don’t Flag Me is the Desperate Plea of the Modern Internet
When you pump up the temperature and pressure high enough, the line between liquid and gas just... vanishes. You end up with something that has the density of a liquid but moves through spaces like a gas. It’s a "supercritical fluid."
There is no "image" for this in a standard 6th-grade science book. It’s a shimmer. It’s a ghost state. It’s used to strip caffeine off coffee beans without using harsh chemicals. It’s also how we might one day clean clothes without water. Understanding these "in-between" states is where the real technology happens.
How to Actually Read State of Matter Visuals
When you are hunting for high-quality solid liquid gas images, you need to look for specific "tells" that indicate accuracy.
- Vibration Indicators: Does the solid have "shiver" marks? If it looks like a brick of frozen marbles, it's a 1950s representation. Modern scientific visualization emphasizes the kinetic energy—the heat—of the particles.
- Intermolecular Forces: Good images often use colors or "glows" to show the invisible bonds. In water (liquid), hydrogen bonds are the "glue" that keeps the droplets together. A good image shows that tension.
- Scale Bars: This is rare, but the best images tell you exactly how much they've zoomed in. We're talking nanometers.
There's a famous study by researchers like Mayer and Gallini regarding how "labeled illustrations" help or hinder learning. They found that if the image is too abstract, the brain just stores it as a "cartoon" and doesn't apply it to the real world. That’s why you might know that "gas particles move fast" but still be surprised when a cold soda can "sweats."
The sweat on the can is a real-life transition from gas to liquid. It’s water vapor in the air losing energy when it hits the cold aluminum and clumping together. If you’ve only ever looked at static solid liquid gas images, you might not make the connection that those "circles" are literally falling out of the air and turning into a puddle on your desk.
The Future of Visualizing Matter
We’re moving past 2D drawings.
Virtual reality (VR) and augmented reality (AR) are changing the game. Companies like Labster or MEL Science are creating simulations where you can literally shrink down and walk through a crystal lattice. You can see the atoms vibrating. You can reach out and "push" a gas molecule to see how it affects pressure.
This is the "pro" version of solid liquid gas images.
Instead of a flat JPEG, we’re looking at dynamic simulations. These allow us to see phase changes in real-time. You can watch the "melting" happen at an atomic level—seeing the precise moment the heat energy overcomes the structural bonds of the solid.
Why This Matters for Technology
If we couldn't visualize these states accurately, we wouldn't have semi-conductors.
Your smartphone relies on our ability to manipulate the "solid" state of silicon by "doping" it with other atoms. We have to visualize exactly where those extra atoms go in the lattice. This isn't just art; it's engineering.
We’re also using these concepts to develop "Smart Materials" that can change state on command. Imagine a liquid that turns into a solid the second you run an electric current through it. It sounds like sci-fi, but it’s just advanced manipulation of state.
Practical Takeaways for Your Next Search
If you're looking for images for a project, a presentation, or just to satisfy your own curiosity, don't settle for the first "three boxes" graphic you find.
- Search for "Phase Diagrams": These are the maps of matter. They show you exactly at what temperature and pressure a substance switches states. It’s much more useful than a simple picture.
- Look for "Molecular Dynamics Simulations": These are often short GIFs or videos. They show the actual movement. A moving image of a liquid is 100x more educational than a static one because the movement defines the state.
- Check the Source: Sites like Phet Interactive Simulations (University of Colorado Boulder) offer the gold standard in accurate, interactive matter visuals.
- Think in 3D: Remember that matter isn't flat. Solids are 3D structures. Gases fill 3D volumes.
Matter is a lot more chaotic and exciting than those dusty old textbooks suggest. Whether it’s the plasma in a lightning bolt or the supercritical fluid in your morning coffee, the "states" of our world are constantly shifting, vibrating, and bumping into one another. Next time you see a set of solid liquid gas images, look for the movement. Look for the energy. That’s where the real science is happening.
Actionable Next Steps:
- Download a simulation tool: Use the PhET "States of Matter" interactive to see how temperature changes particle behavior in real-time.
- Observe a Phase Change: Put a cup of water in the freezer and check it every 20 minutes; try to spot the exact moment the "liquid" lattice begins to lock into a "solid" crystal.
- Search specifically for "Amorphous vs Crystalline": This will show you the visual difference between things like window glass and table salt, which is a major "missing link" in basic matter education.