Think about your body for a second. It's basically a giant construction site that never sleeps. While we obsess over DNA—the "blueprints"—there’s a middleman doing all the heavy lifting. That’s ribosomal RNA. Honestly, if DNA is the architect, rRNA function is the literal power tool that builds the house. Without it, your genetic code is just a useless pile of instructions gathering dust in the nucleus.
We’re taught in high school that DNA makes RNA and RNA makes protein. It sounds so clean. So linear. But the reality is a beautiful, chaotic mess of molecular machinery. Ribosomal RNA (rRNA) is the most abundant type of RNA in your cells, making up about 80% of the total RNA. That’s a staggering amount. It’s not just a "helper" molecule; it is the physical heart of the ribosome.
What is the function of rRNA in simple terms?
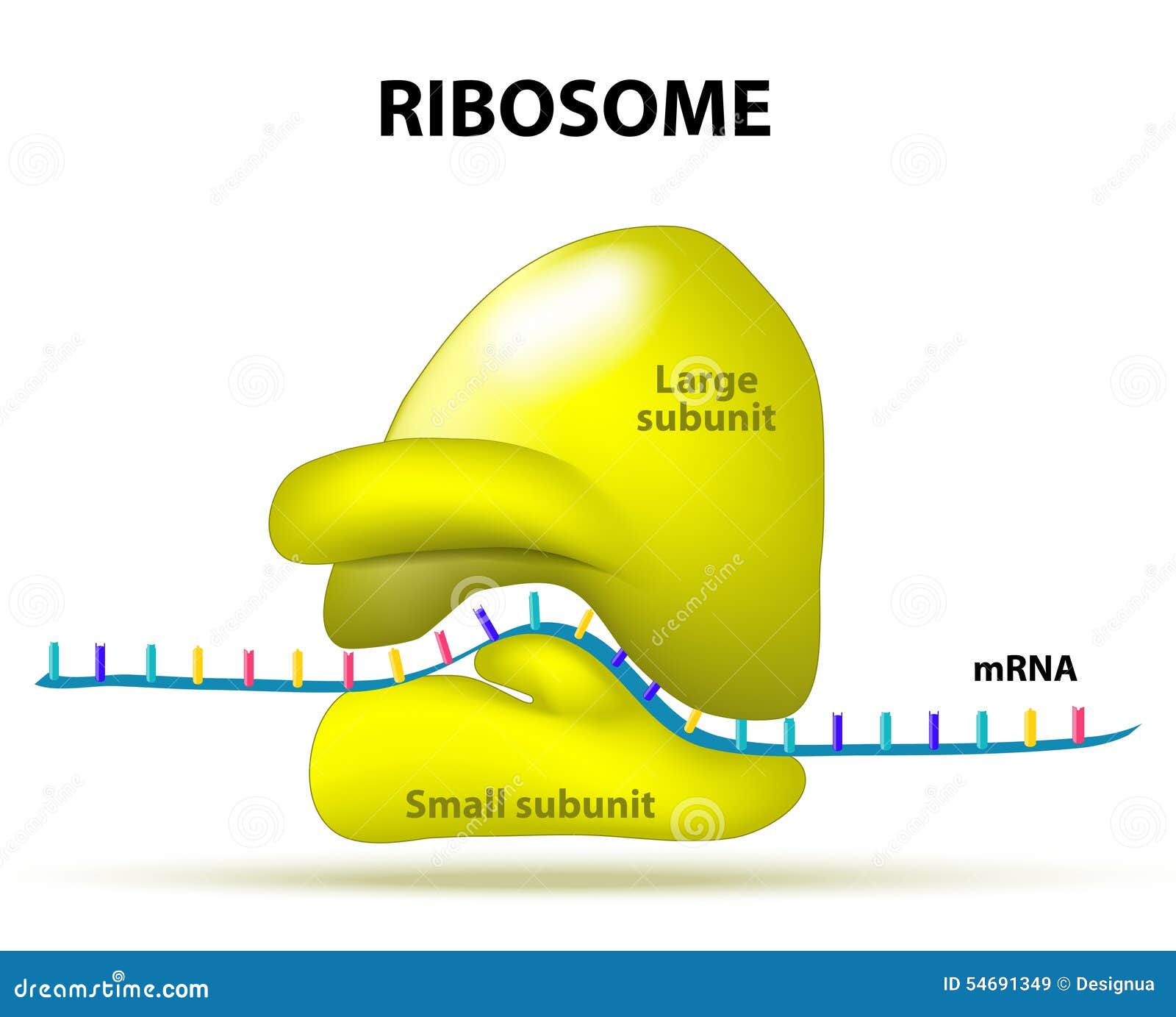
Most people think of ribosomes as solid little dots on a diagram. They aren't. They are complex "ribonucleoproteins." Essentially, they are a mix of protein and rRNA. But here is the kicker: the rRNA isn't just a scaffold. It’s the catalyst.
Inside the ribosome, the rRNA function is to act as a ribozyme. That’s an enzyme made of RNA instead of protein. It physically stitches amino acids together. This happens at a specific spot called the peptidyl transferase center. Imagine a seamstress. The mRNA is the pattern, the tRNA brings the fabric scraps (amino acids), and the rRNA is the needle and thread doing the actual sewing.
📖 Related: How to Accept Dying Without Losing Your Mind: What Most People Get Wrong
The subunits and why they matter
Ribosomes come in two main pieces: the large subunit and the small subunit. In humans (eukaryotes), we call these the 60S and 40S subunits.
The small subunit is the "reader." It makes sure the transfer RNA (tRNA) matches up perfectly with the messenger RNA (mRNA) coming from your DNA. If the match is wrong, the process stalls. It's a quality control system that is nearly foolproof.
Then you have the large subunit. This is where the heavy chemistry happens. The rRNA in the large subunit facilitates the peptide bond formation. It’s fast. It’s precise. And it’s the only reason you have hair, muscle, and enzymes to digest your lunch.
It’s older than life as we know it
Biologists like Carl Woese spent years looking at rRNA, specifically the 16S and 18S strands. Why? Because these sequences change so slowly over millions of years that they act like a molecular clock.
If you want to know how a human is related to a mushroom or a bacterium, you don't look at "cool" genes like those for eye color. You look at rRNA. It’s so fundamental to life that evolution rarely messes with it. If the rRNA function breaks, the organism dies immediately. There's no room for "trying something new" here.
This is why rRNA is the gold standard for phylogenetics. By sequencing the rRNA of different species, scientists mapped the Three Domains of Life: Archaea, Bacteria, and Eukarya. It literally redrew the tree of life.
Why your antibiotics target rRNA
Here is a wild fact: many of the antibiotics you take, like erythromycin or tetracycline, work by attacking bacterial rRNA.
Bacteria have slightly different ribosomes than humans (70S vs 80S). These drugs are designed to gum up the works of the bacterial rRNA specifically. It’s like putting a metal rod into the gears of a specific engine. The bacteria can no longer make proteins. They can't build cell walls. They can't reproduce. Meanwhile, your human ribosomes keep humming along because their rRNA structure is just different enough to ignore the drug.
💡 You might also like: Does Alcohol Stop Period Flow? What Actually Happens to Your Cycle
The secret life of the nucleolus
Where does all this rRNA come from? It isn't just floating around. It’s born in a dark, dense spot inside your nucleus called the nucleolus.
The nucleolus is basically an rRNA factory. Huge stretches of DNA contain "ribosomal DNA" repeats. Your cell turns these into long precursor rRNA strands, which then get chopped up, folded, and packaged with proteins. It’s a massive energy sink. In fact, a rapidly growing cell can spend up to 60% of its total energy just making ribosomes.
Why so much? Because a single cell might need ten million ribosomes to keep up with protein demand. If you're a muscle cell trying to repair after a workout, or a skin cell regenerating after a scrape, your rRNA production goes into overdrive.
Misconceptions about "Junk" RNA
For a long time, people thought protein-coding DNA was the only thing that mattered. Everything else was "junk." We now know that's total nonsense.
The rRNA function is more active than we ever imagined. It doesn't just sit there. Recent research suggests that ribosomes might be "specialized." This means a ribosome in your brain might have slightly different rRNA components than a ribosome in your liver. This "Ribosome Filter Hypothesis" suggests that rRNA might actually help decide which mRNAs get translated and which don't. It's a layer of control we are only just beginning to understand.
What happens when rRNA fails?
When things go wrong with ribosomal RNA or the proteins that support it, we see a group of diseases called ribosomopathies.
Take Diamond-Blackfan anemia, for example. It’s a rare disorder where the body can’t produce enough red blood cells. The cause? Mutations in the proteins that help assemble the rRNA into a working ribosome. Or Treacher Collins syndrome, which affects the development of bones and tissues in the face.
These diseases prove that rRNA function is the foundation of human development. If the "sewing machine" is broken, it doesn't matter how good the fabric or the pattern is. The whole system collapses.
The link to aging and cancer
Cancer cells are obsessed with rRNA. Since cancer is essentially uncontrolled growth, these cells need an endless supply of proteins. Consequently, they often have enlarged nucleoli and jacked-up rRNA production.
On the flip side, some researchers believe that slowing down ribosome biogenesis might actually slow down aging. It’s a "less is more" strategy. By forcing the cell to be more selective and efficient with its protein production, you might reduce the "molecular trash" (misfolded proteins) that contributes to aging.
Practical takeaways for your health
So, what does this mean for you, the non-scientist? Honestly, it’s about understanding the metabolic cost of your body's maintenance.
- Nutrition matters for rRNA: Making rRNA requires specific building blocks, including phosphorus and nitrogen. A starved cell can't maintain its ribosome count.
- Stress impacts production: Environmental stress can cause "ribosomal stress," where the cell stops making rRNA to save energy. This is a survival mechanism, but long-term, it's why chronic stress makes you feel physically depleted.
- Antibiotic awareness: Understanding that antibiotics target the "bacterial version" of your own machinery helps explain why they shouldn't be overused. You want to kill the bad ribosomes, not encourage the bacteria to evolve "armored" rRNA that drugs can't touch.
The rRNA function is the silent engine of life. It is the bridge between the digital information of your genome and the physical reality of your body. Next time you look at your hand or take a breath, remember the trillions of tiny RNA "needles" inside you, stitching together the proteins that make it all possible.
To better understand your own cellular health, consider looking into "proteostasis"—the balance of protein production and recycling in your body. You can also track your metabolic health through routine blood work that monitors markers like C-reactive protein, which can indicate if your cells (and their ribosomes) are under systemic inflammatory stress.