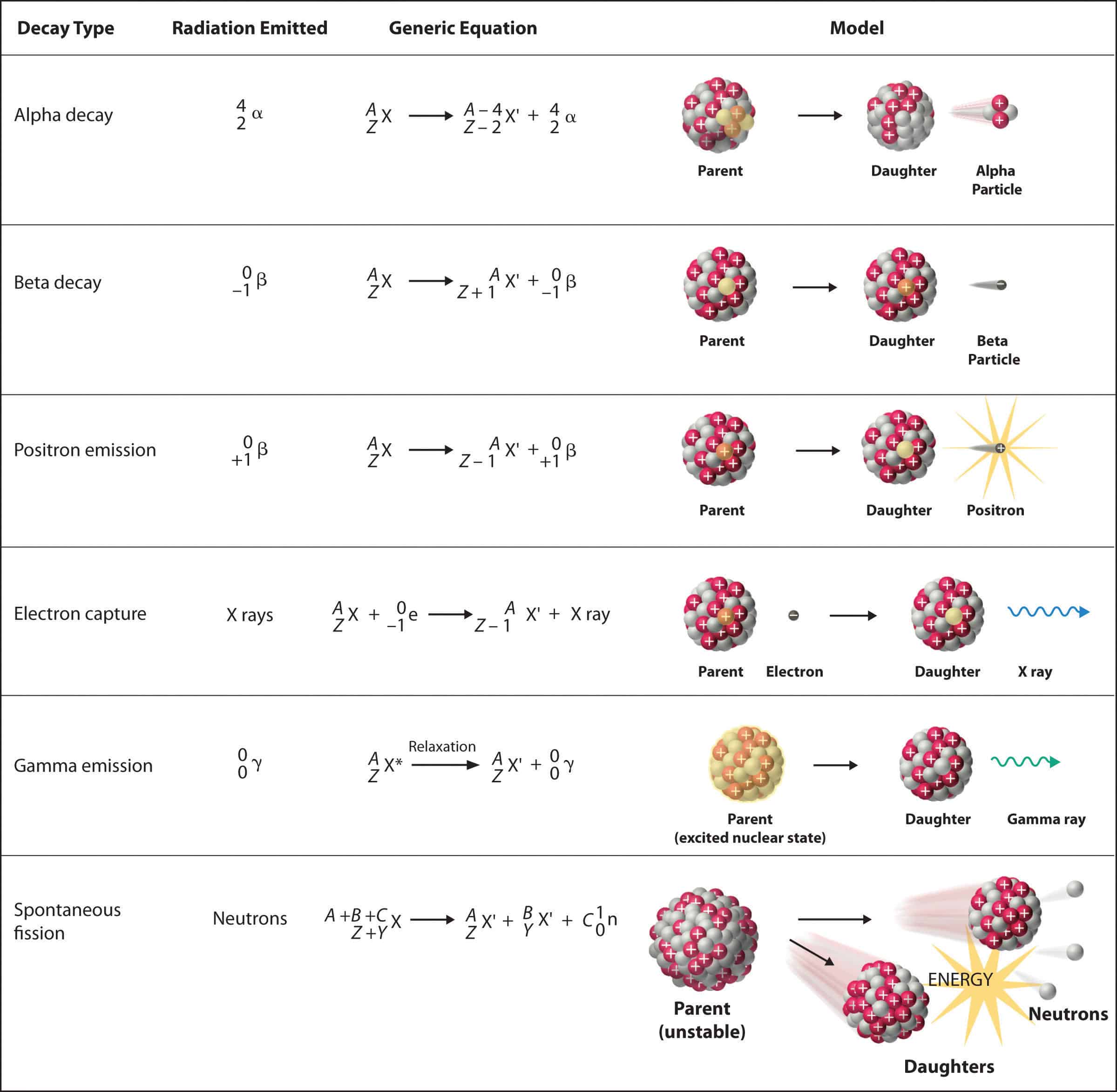
Ever stared at a periodic table and wondered why some elements just can’t stay still? It’s kinda wild when you think about it. Atoms, the very building blocks of your morning coffee and your smartphone, can literally fall apart. This isn't just a random mess, though. There is a very specific, almost poetic bookkeeping involved. We call these radioactive decay nuclear equations. If you can balance a checkbook—or at least track how much money you spent on takeout last week—you can master these equations. It’s all about conservation. You don't just lose stuff into the void. It just changes shape.
Most people freak out when they see Greek letters like alpha ($\alpha$) or beta ($\beta$). Don't. They’re just labels for the "trash" an unstable atom throws out to feel better. When an atom's nucleus has too much energy or a bad ratio of protons to neutrons, it spits something out. That "spitting out" is the decay. The equation is just the "before and after" photo of that event.
The Golden Rules of Nuclear Accounting
You've gotta remember two numbers: the atomic number and the mass number. That’s the whole game. In any radioactive decay nuclear equations, the total mass on the left must equal the total mass on the right. Same goes for the charge. If you start with 92 protons, you better end with 92 "units" of charge on the other side, even if they’re split between two different particles.
Think of the nucleus like a shaky tower of Jenga blocks. If it’s too tall (too much mass) or too lopsided (too many neutrons), a piece is going to fly out. When that piece leaves, the tower becomes something else. That’s transmutation. It’s the literal alchemy that medieval scientists dreamed about, but instead of magic wands, we’re using the weak and strong nuclear forces.
Ernest Rutherford, the guy who basically discovered the nucleus, realized early on that this wasn't just chemical. In chemical reactions, atoms swap partners. In nuclear decay, the atoms themselves transform. It’s fundamental. It’s gritty. And it follows the math every single time.
Alpha Decay: The Heavy Lifter
Alpha decay is the "big move." Imagine an atom is carrying a heavy suitcase and decides it’s too much work, so it hurls a chunk of it away. That chunk is an alpha particle. Specifically, it’s two protons and two neutrons. If you're looking at your periodic table, that's just a Helium-4 nucleus.
When an element like Uranium-238 undergoes alpha decay, it loses 4 from its mass and 2 from its atomic number.
👉 See also: Samsung 11 inch tablet: What Most People Get Wrong
$$^{238}{92}\text{U} \rightarrow ^{234}{90}\text{Th} + ^4_2\text{He}$$
See that? 238 becomes 234 + 4. 92 becomes 90 + 2. It’s clean. Thorium is born from the "ashes" of Uranium. This usually happens in the heavyweights of the periodic table—the elements with atomic numbers higher than 82. Lead is basically the retirement home for these heavy elements. Once they hit lead, they usually stop shaking and stay put.
Beta Decay: The Neutron’s Identity Crisis
Beta decay is weirder. It’s honestly a bit trippy. Imagine a neutron sitting in the nucleus. It decides it would rather be a proton. To make that happen, it spits out an electron (the beta particle) and a tiny, ghostly thing called an antineutrino.
Because an electron has a negative charge, losing it actually increases the atomic number of the atom. You’ve turned a neutral particle into a positive one. The mass doesn't really change because electrons are incredibly light, but the identity of the element jumps one spot to the right on the periodic table. Carbon-14 does this to become Nitrogen-14. This is the heartbeat of carbon dating. Without this specific equation, we wouldn't know how old those ancient bones in the museum actually are.
What about Beta Plus?
There’s a sibling to this called Positron Emission. This is where a proton turns into a neutron and spits out a positron (a "positive electron"). It’s the basis for PET scans in hospitals. If you’ve ever had one, you’ve had radioactive tracers inside you performing these exact equations in real-time so doctors can see your metabolic activity. Science is cool, but it’s also incredibly practical.
Gamma Decay: The Energy Purge
Then there’s Gamma. Gamma is the odd one out because it doesn't change the element. No protons are lost. No neutrons change. It’s just pure, high-frequency electromagnetic radiation.
Imagine you just finished a high-intensity workout. You're the same person, but you're radiating heat. That’s basically what an excited nucleus does with a gamma ray. It’s just shedding excess energy to get down to a "ground state." In radioactive decay nuclear equations for gamma, you’ll often see a little "m" (for metastable) or an asterisk next to the element symbol, which then disappears on the right side of the arrow.
Why Do We Actually Care?
It’s easy to think this is just for textbooks. Wrong.
Smoke detectors in your hallway? Most of them use Americium-241, an alpha emitter. The alpha particles ionize the air, creating a tiny current. When smoke gets in the way, it breaks that current, and the alarm screams at you. That’s a nuclear equation saving your life while you sleep.
Nuclear power? It's all about managing these rates of decay and fission. Even the heat inside the Earth—the stuff that moves tectonic plates and fuels volcanoes—comes largely from the decay of Potassium-40, Uranium, and Thorium in the mantle. We live on a giant nuclear-heated rock.
Common Pitfalls in Balancing Equations
- Forgetting the Electron: In beta decay, people often forget to write the -1 in the bottom spot for the electron. Remember: $0 - (-1) = 1$. That’s why the atomic number goes up.
- The Neutrino Ghost: In advanced physics, you have to account for neutrinos to conserve lepton number. For a general chemistry test? You might get away with ignoring them, but a real pro always remembers the ghosts in the machine.
- Mass vs. Weight: We are talking about mass numbers (protons + neutrons), not the decimal-heavy atomic weights you see on the periodic table. Use the whole numbers.
Real-World Nuance: The Decay Chain
Elements don't just decay once and quit. It’s a marathon. Uranium-238 goes through a whole "decay chain," hitting Thorium, Protactinium, back to Uranium (a different isotope), then eventually through Radium and Radon before finally finding peace as Lead-206.
If you have Radon gas in your basement, you’re dealing with a middle-child of a long nuclear family tree. It’s a gas, so it seeps through cracks in the foundation. Understanding the equations helps engineers design better ventilation to keep that stuff out of your lungs.
Step-by-Step: Writing Your Own Equation
- Identify the Parent: Start with the unstable isotope. Write its symbol with the mass number on top and atomic number on the bottom.
- Pick the Decay Type: Is it alpha, beta, or gamma?
- Subtract (or Add): For alpha, subtract 4 from the top and 2 from the bottom. For beta minus, keep the top the same and add 1 to the bottom.
- Find the New Identity: Look at the new atomic number. Find that number on the periodic table. That’s your "daughter" element.
- Check Your Math: Does $Left = Right$? If yes, you’re golden.
Actionable Insights for Learners
If you're trying to master this for a class or just for your own curiosity, stop trying to memorize every single isotope. Instead, focus on the particles. If you know that an alpha particle is $^4_2\text{He}$ and a beta particle is $^0_{-1}\text{e}$, you can solve literally any equation thrown at you.
- Download a High-Res Periodic Table: One that shows the common decay modes for unstable isotopes.
- Practice with Carbon-14: It’s the most famous example for a reason. Write out the beta decay until you can do it in your sleep.
- Check for Conservation: Always do a quick "top row" and "bottom row" sum check. It takes two seconds and catches 90% of errors.
Nuclear physics isn't some dark art. It's just nature trying to find a balance. Every time an atom decays, it's just trying to find a more stable way to exist. We’re just the accountants tracking the changes.
To take this further, start looking into half-lives. Once you know how an atom decays via these equations, the next logical step is figuring out how long it takes for a pile of those atoms to disappear. That’s where the math gets really interesting and where we start talking about everything from medical dose timing to the age of the solar system.