Walk into any high school science lab and you'll see a periodic table staring back at you. It’s the holy grail of matter. But here’s the thing: most of the stuff you actually interact with isn't just a pile of gold or a cloud of oxygen. It’s more complicated. Most things are joined at the hip. When we talk about pure compounds, we are talking about substances that have decided to stop being single and have committed to a chemical bond.
It’s weirdly specific.
A pure compound isn't just "stuff mixed together." That’s a mixture. If you throw sand into a bucket of water, you’ve got a mess, not a compound. To be a pure compound, those atoms have to be legally married in the eyes of physics. They are held together by chemical bonds—covalent or ionic—and they don’t just come apart because you shook the jar.
Defining Pure Compounds Without the Textbook Fluff
So, what are pure compounds? Basically, they are substances made up of two or more different elements that are chemically joined in a fixed ratio. That "fixed ratio" part is the secret sauce. Take water ($H_{2}O$). It is always two hydrogens and one oxygen. If you try to get fancy and add another oxygen, you don't have "extra-strength water." You have hydrogen peroxide ($H_{2}O_{2}$), which will bleach your hair or sting your cuts.
The identity of a compound is tied to this ratio.
Nature is surprisingly rigid about this. Joseph Proust, a French chemist back in the late 1700s, figured this out and called it the Law of Definite Proportions. He realized that a chemical compound always contains exactly the same proportion of elements by mass. It doesn't matter if the water came from a glacier in Antarctica or a tap in New Jersey; the ratio of hydrogen to oxygen is identical.
Why Pure Compounds Act So Different From Their Parents
This is the part that always trips people up. When elements join to form a compound, they lose their individual "personalities."
Take common table salt, or sodium chloride ($NaCl$). Sodium is a soft, silvery metal that literally explodes if it touches water. Chlorine is a greenish, toxic gas used in chemical warfare. You wouldn't want either at your dinner party. But when they bond? They form a stable, white crystal that you sprinkle on your fries.
The properties of the compound are entirely new.
They have their own specific melting points, boiling points, and densities. This is how scientists identify them. If you have a clear liquid and you aren't sure if it’s pure water, you check the boiling point. At sea level, it better be exactly 100°C. If it’s 102°C, something else is in there. It’s no longer a pure compound; it’s a solution.
The Two Main Tribes: Covalent and Ionic
Not all bonds are created equal. Atoms find different ways to stick together, and this changes how the resulting pure compound behaves in the real world.
Covalent compounds are the sharers. These usually happen between non-metals. Atoms sit close and share electrons to feel stable. Think of carbon dioxide ($CO_{2}$) or methane ($CH_{4}$). They tend to have lower melting points. Many are gases or liquids at room temperature.
Ionic compounds are more about the "theft" and "attraction" model. One atom gives up an electron, another takes it, and now they are oppositely charged. They stick together like powerful magnets. This is your salt, your baking soda (sodium bicarbonate), and your calcium carbonate (limestone). They usually form crystalline solids and have incredibly high melting points because that magnetic-like pull is hard to break.
Common Pure Compounds You Use Every Day
Sometimes we get so caught up in the "pure" part that we forget how common these are. We aren't just talking about stuff in a glass vial in a lab.
- Sucrose ($C_{12}H_{22}O_{11}$): That’s just table sugar. It’s a complex arrangement of carbon, hydrogen, and oxygen. Even though it looks like a simple powder, every single grain is a massive collection of these identical molecules.
- Methane ($CH_{4}$): The primary component of natural gas. It’s a simple pure compound, but it’s the backbone of how we heat half the homes in the country.
- Ammonia ($NH_{3}$): Used in everything from window cleaner to fertilizer. It’s a pungent reminder that three hydrogens and one nitrogen have a very specific, nose-stinging identity.
- Silicon Dioxide ($SiO_{2}$): This is the main ingredient in quartz and glass. It’s a "network solid," meaning the atoms are bonded in a continuous web.
The Purity Paradox
Here is a bit of honesty: finding a truly "pure" compound in the wild is actually pretty rare.
📖 Related: Why Did the Hindenburg Explode? What Most People Get Wrong
Earth is a messy place. Water in a river isn't a pure compound; it’s a mixture of $H_{2}O$, dissolved minerals, fish poop, and oxygen gas. To get a truly pure compound, humans usually have to intervene. We use distillation, filtration, or recrystallization to strip away the "extras" until only the bonded molecules remain.
In the world of semi-conductors—the chips in your phone—purity is everything. If a crystal of silicon has even a few rogue atoms of something else, the whole thing might fail. We spend billions of dollars to create "pure" environments because the behavior of a compound is only predictable when it's actually alone.
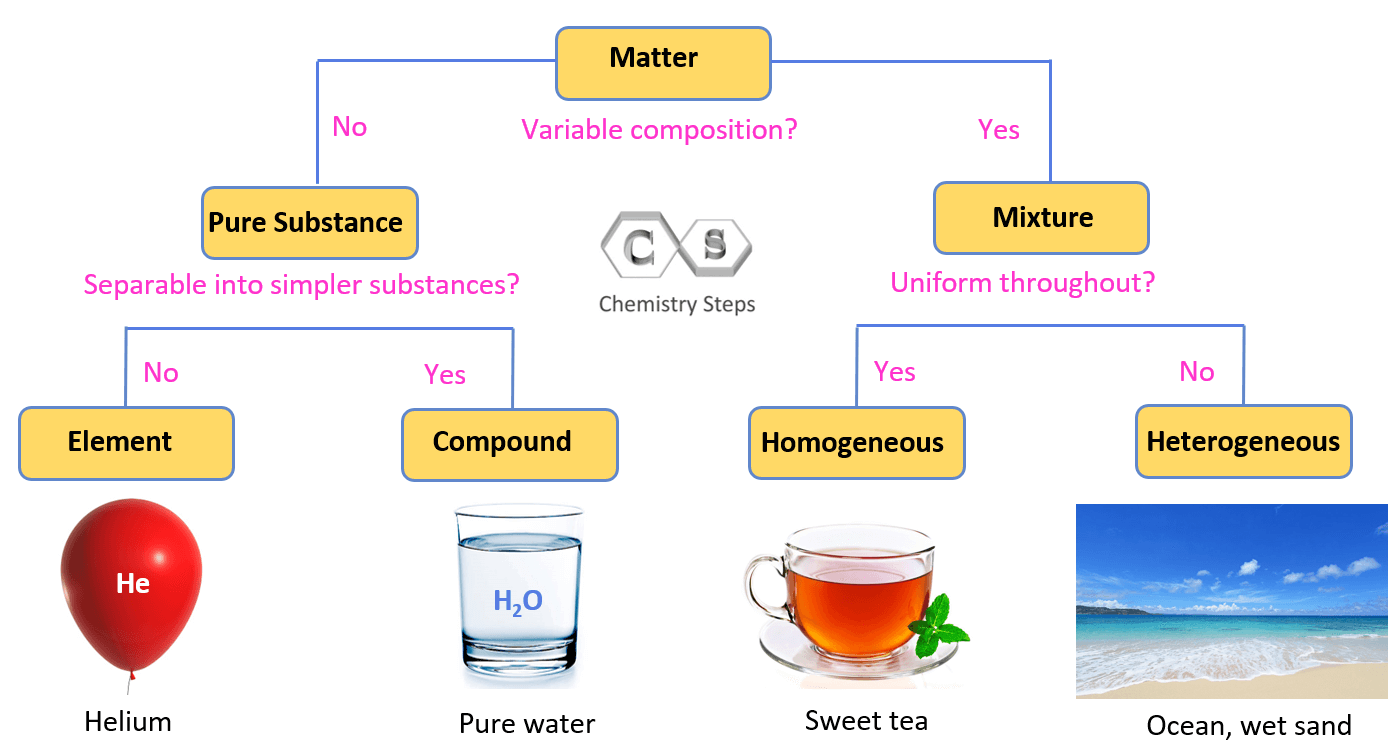
Is an Element a Pure Compound?
Short answer: No.
Long answer: They are both "pure substances," but they aren't the same thing.
An element is made of only one type of atom. Gold is just gold. Oxygen gas ($O_{2}$) is just oxygen. Even though $O_{2}$ has two atoms bonded together, it’s not a compound because those atoms are the same element. To be a compound, you need a "heterogeneous" mix of elements—different types of atoms working together.
Why This Matters for the Future of Tech and Health
We are currently in an era where we are "designing" new pure compounds to solve specific problems. Look at the pharmaceutical industry. Every time a company like Pfizer or Moderna develops a new drug, they are essentially searching for a specific pure compound that fits into a protein receptor like a key into a lock.
If the ratio is off, the drug doesn't work. If the bond is in the wrong place, it might be toxic.
📖 Related: Why You Can't Skip YouTube Ads Anymore and How the Tech Actually Works
In the energy sector, we are looking at metal-organic frameworks (MOFs). These are highly porous pure compounds that can act like sponges for carbon dioxide. By understanding the geometry of how these atoms bond, we can literally build "cages" to trap greenhouse gases at the molecular level.
It’s not just "old" chemistry. It’s the frontier of materials science.
How to Identify a Pure Compound Yourself
You don't need a million-dollar lab to tell the difference between a mixture and a compound, though it helps. Here is the mental checklist:
- Can you separate it physically? If you can use a magnet, a filter, or just pick it apart with tweezers, it's a mixture. You can't "filter" the hydrogen out of water. You need a chemical reaction (like electrolysis) to break those bonds.
- Does it have a "sharp" melting point? Pure compounds melt at a specific temperature. Mixtures (like wax or butter) tend to soften over a range of temperatures because different parts are melting at different times.
- Is it uniform? This is tricky because some mixtures (like salt water) look uniform. But a pure compound is uniform at the molecular level.
Moving Toward Molecular Precision
Understanding pure compounds is about moving past the idea that the world is just a "soup" of matter. It’s structured. It’s mathematical. Every time you breathe out $CO_{2}$, you are participating in a cycle of creating a specific, bonded unit of matter that has existed since the dawn of the universe.
If you're looking to dive deeper into this, your next step should be looking into stoichiometry. It sounds intimidating, but it’s just the math of how these compounds react. Once you know the "recipe" of a compound, you can predict exactly how much of each element you need to create it.
You might also want to explore the Chirality of compounds. Some compounds have the exact same formula—the same number of atoms—but they are "left-handed" or "right-handed" versions of each other. In the world of medicine, one version might cure you, while the other does nothing or causes harm. It's a fascinating rabbit hole that shows just how much the "purity" of a compound depends on its physical shape, not just its ingredients.
Start by checking the labels on your household cleaners or food. Look for the chemical names. Try to look up their formulas. When you see "Sodium Bicarbonate," realize you're looking at a specific, repeatable miracle of atomic bonding.