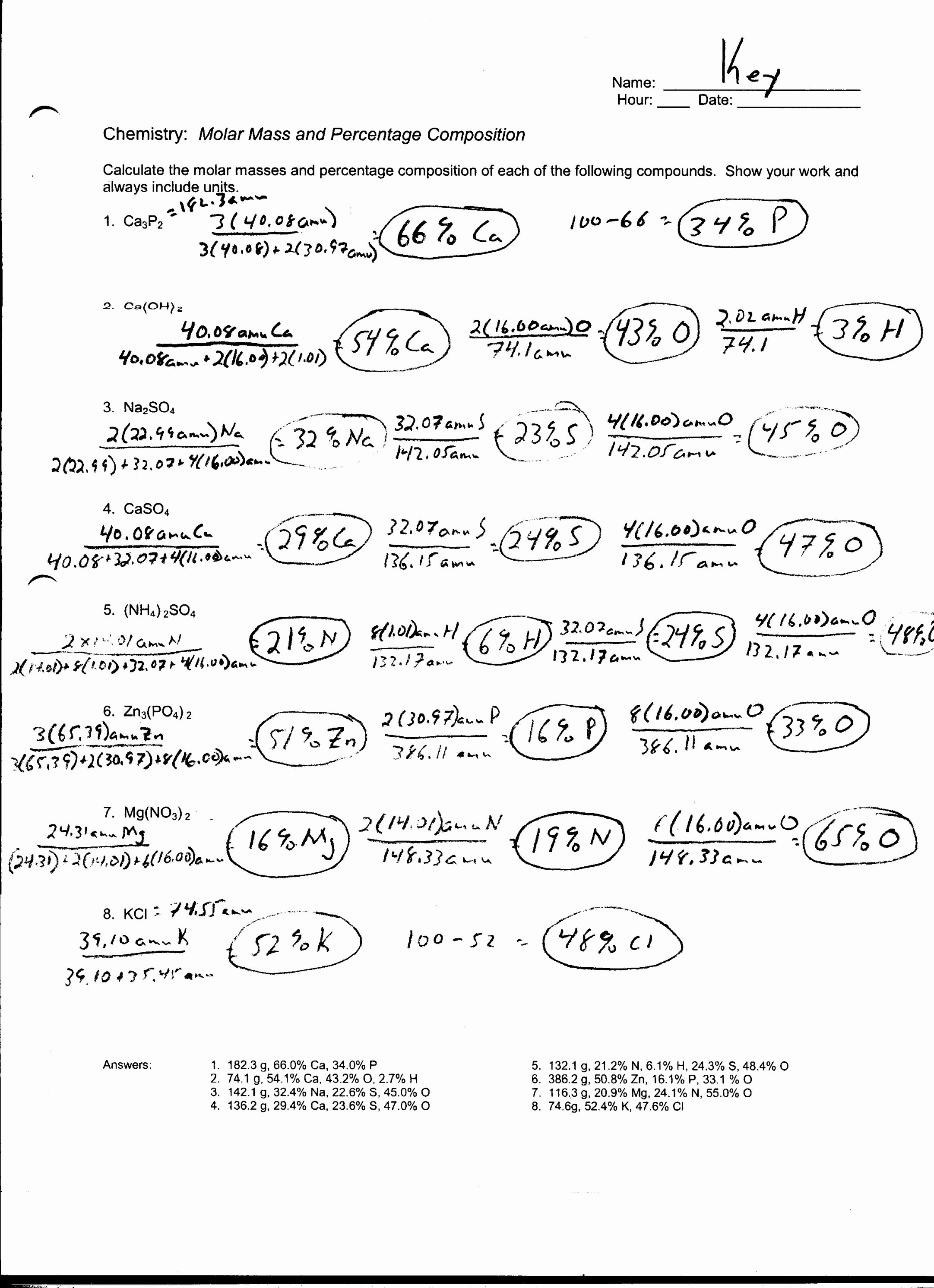
You’re sitting in lab. The instructor hands you a vial of white powder and tells you to calculate how many moles of sodium chloride are in that 5.8-gram pile. Suddenly, your brain freezes. You know the formula is $NaCl$. You know you need the periodic table. But the actual math? It feels like trying to translate a dead language while underwater. Honestly, almost everyone hits this wall at some point in Gen Chem.
Mastering molar mass practice problems isn't just about passing a quiz. It is the fundamental "gatekeeper" skill. If you can’t calculate molar mass, you can’t do stoichiometry. If you can’t do stoichiometry, you can’t predict how much product a reaction will make. Basically, you're stuck. But here is the thing: it’s actually just glorified addition.
What People Get Wrong About the Periodic Table
Most students look at the periodic table and see a mess of numbers. They grab the atomic number—the small whole number at the top—instead of the average atomic mass at the bottom. Big mistake. The atomic number tells you how many protons are in the atom. The molar mass, usually expressed in grams per mole ($g/mol$), is that decimal-heavy number at the bottom.
Think of it like this. If you’re weighing a dozen eggs, you don't care about the "egg ID number." You care about how much the eggs actually weigh. For an element like Oxygen, that number is 15.999. In most intro classes, your teacher is fine with you rounding that to 16.00, but always check their syllabus first. Some professors are sticklers for those extra decimals.
Walking Through a Basic Problem
Let’s look at Water ($H_2O$). It’s the classic starting point for any set of molar mass practice problems. You have two Hydrogen atoms and one Oxygen atom.
Hydrogen has an atomic mass of roughly 1.008. Since there are two of them, you multiply: $1.008 \times 2 = 2.016$.
Oxygen is 15.999.
Add them together: $2.016 + 15.999 = 18.015\ g/mol$.
Simple, right? It gets trickier when you hit parentheses.
Take Magnesium Nitrate, $Mg(NO_3)_2$. Those little numbers—the subscripts—are where the wheels usually fall off. That "2" outside the parentheses means you have two of everything inside. That's two Nitrogens and six Oxygens. If you forget to distribute that number, your entire calculation is toast.
Why Units Actually Matter (Seriously)
Don’t just write "18." Writing the unit $g/mol$ is vital. It’s a conversion factor. It tells you that if you have exactly 6.022 x 10²³ molecules of water (Avogadro's number), that pile will weigh exactly 18.015 grams.
Chemistry is basically just a series of unit conversions. If you treat your units like a map, you’ll rarely get lost. Amedeo Avogadro, the Italian scientist who laid the groundwork for this, didn't just come up with his constant for fun. He realized there had to be a bridge between the microscopic world of atoms and the macroscopic world of the stuff we can actually see and weigh in a beaker. Molar mass is that bridge.
Mastering Intermediate Molar Mass Practice Problems
Once you’ve got the basics down, you’ll encounter hydrates. These are the "boss level" of basic molar mass. A hydrate is a salt that has water molecules physically trapped inside its crystal lattice. You’ll see them written with a little dot, like $CuSO_4 \cdot 5H_2O$ (Copper(II) Sulfate Pentahydrate).
That dot does not mean multiplication.
I’ll repeat that because it’s the most common error in molar mass practice problems. The dot means "and." You calculate the mass of the $CuSO_4$ and then add the mass of five water molecules to it.
Let's crunch the numbers for Copper(II) Sulfate Pentahydrate:
- Copper ($Cu$): 63.55
- Sulfur ($S$): 32.06
- Oxygen ($O_4$): $16.00 \times 4 = 64.00$
- Water ($5H_2O$): $5 \times 18.02 = 90.10$
Total: $63.55 + 32.06 + 64.00 + 90.10 = 249.71\ g/mol$.
If you accidentally multiplied those masses, you’d end up with a number so large it wouldn't even make sense in the context of the physical world. Always do a "sanity check" on your answers. If you’re calculating the molar mass of a small molecule and you get 5,000, you probably hit a wrong button on your calculator or misinterpreted a symbol.
The Reality of Significant Figures
Sig figs are the bane of every chemistry student's existence. But they exist for a reason. They reflect the precision of your measurements. When working through molar mass practice problems, usually, the rule is to match the number of decimal places provided by your periodic table. If your table gives you four decimal places, use them. Don't get lazy and round 35.453 (Chlorine) to 35. It will mess up your results when you start doing multi-step stoichiometry later on.
Real-World Application: Why This Isn't Just "Busy Work"
In pharmaceutical labs, being off by a few milligrams can be the difference between a life-saving dose and a toxic one. When chemists at companies like Pfizer or Moderna are synthesizing new compounds, they rely on molar mass to calculate "percent yield."
If you know you should have created 100 grams of a drug based on your molar mass calculations, but you only got 80 grams, you have an 80% yield. Understanding where those missing 20 grams went requires a deep dive into the reaction mechanics, but it all starts with that initial molar mass calculation.
It’s also huge in environmental science. When testing for lead in water, technicians convert the mass of the lead found into moles to compare it against safety standards set by the EPA. Without an accurate molar mass for lead (207.2 $g/mol$), those safety checks are useless.
How to Get Faster (And More Accurate)
Stop trying to do it all in your head.
Write out each element. Write the count. Write the atomic mass. It takes an extra ten seconds, but it prevents the "dumb" mistakes that tank grades.
- Step 1: List every element in the compound.
- Step 2: Count the atoms. Look at the subscripts and remember to multiply through parentheses.
- Step 3: Find the atomic mass on a reliable periodic table (the one provided for your exam is best).
- Step 4: Multiply the mass by the count for each element.
- Step 5: Sum the totals.
- Step 6: Label with $g/mol$.
If you follow that workflow every single time, you'll find that molar mass practice problems become second nature. You’ll start seeing patterns. You’ll eventually memorize that Carbon is 12.01 and Oxygen is 16.00 because you use them so often.
Actionable Next Steps
Don't just read about it. Chemistry is a "doing" subject.
👉 See also: Talking watch for the blind: Why the simplest tech still wins in a screen-heavy world
- Grab a pencil and paper. No, seriously. Don't use a notes app. The tactile act of writing helps your brain process the math.
- Download a standard periodic table. Use the one from the Royal Society of Chemistry or a similar reputable source so you're used to high-precision numbers.
- Start with the "Big 5" compounds. Calculate the molar mass for:
- Sulfuric Acid ($H_2SO_4$)
- Glucose ($C_6H_{12}O_6$)
- Calcium Carbonate ($CaCO_3$)
- Ammonium Phosphate ($(NH_4)_3PO_4$)
- Iron(III) Oxide ($Fe_2O_3$)
- Verify your answers. If you get stuck on the Ammonium Phosphate, remember: that 3 outside the parentheses applies to both the Nitrogen and the Hydrogen. You have 3 Nitrogens and 12 Hydrogens.
Once you can nail those five without checking your notes, you're ready for the more complex stoichiometry problems that usually follow this unit. Chemistry is a pyramid; make sure your base—this skill—is solid.