Look at the device you're holding. Honestly, it's a miracle of chemistry. That screen, the battery, the tiny circuits—they all rely on a very specific dance between different types of elements. If you’ve ever stared at a periodic table and felt like it was just a wall of confusing symbols, you aren't alone. But it's basically the "ingredients list" for the entire universe. Understanding metals metalloids and nonmetals properties isn't just for passing a high school chemistry quiz; it’s about knowing why your phone doesn't melt in your hand and why we breathe oxygen instead of, say, nitrogen-infused gold.
Most of the table is metal. It’s the dominant neighborhood. Then you’ve got the nonmetals, the weird, gassy, or brittle stuff on the right. And stuck right in the middle, like a bridge between two worlds, are the metalloids. They are the "chameleons" of the elemental world.
📖 Related: Why a Radio and CD Player with Bluetooth is the Unlikely Hero of Modern Home Audio
The Heavy Hitters: Why Metals Run the World
Metals are the extroverts of the periodic table. They love to give away electrons. Because they are so generous with their subatomic particles, they end up with these very specific traits that we use every single day. Think about Copper ($Cu$). It's the gold standard for wiring. Why? Because its electrons are "delocalized." They just wander around, which allows electricity to flow like water through a pipe.
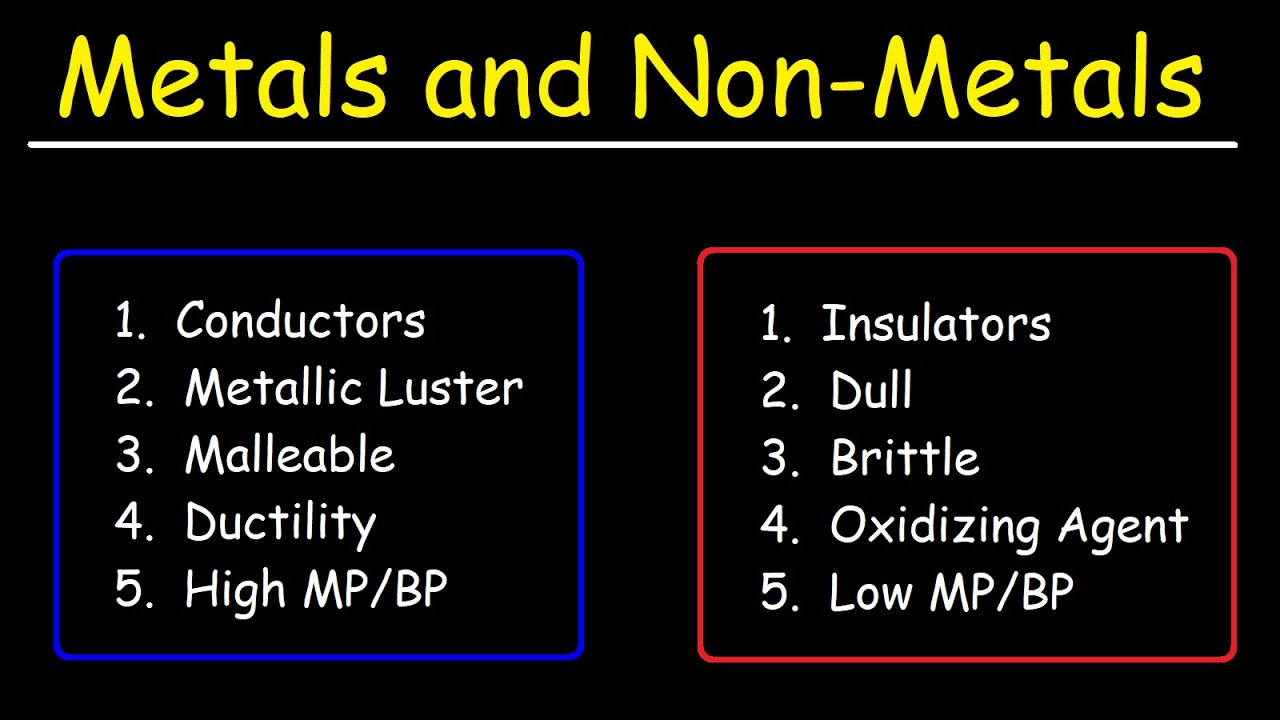
When we talk about metals, we usually think of things that are shiny. Scientists call this "luster." It's not just for looks; it's about how the surface reflects light. But beauty is only skin deep. The real magic of metals lies in two words that sound like they belong in a blacksmith’s shop: malleability and ductility.
Malleability means you can take a hammer to it and it turns into a sheet rather than shattering into a million pieces. Ductility is the ability to pull it into a thin wire. Try doing that with a piece of coal (carbon) and you'll just end up with a mess. Metals can handle the pressure because their atoms are arranged in a regular, layered structure that can slide past each other without breaking the metallic bond.
It's not all about construction and wires, though. Mercury ($Hg$) is the rebel here. It’s a metal, but it’s a liquid at room temperature. It defies the "solid" rule that most people associate with metals. Then you have the Alkali metals like Sodium ($Na$). You’ve probably seen those videos where someone drops a chunk of Sodium into water and it explodes. That’s because these metals are so desperate to get rid of that one outer electron that they react violently with almost anything.
The Quiet Power of Nonmetals
If metals are the extroverts, nonmetals are the introverts. They don't want to give anything away. In fact, they usually want to take electrons. This makes them the polar opposite of metals in almost every way.
Most nonmetals are gases at room temperature. Think Nitrogen ($N$), Oxygen ($O$), and Fluorine ($F$). If they are solid, like Sulfur ($S$) or Phosphorus ($P$), they are incredibly brittle. You can’t hammer Sulfur into a sheet. It’ll just turn into a yellow powder. They are also terrible at conducting heat or electricity. They are the insulators of the world. This is why the handles on your frying pans aren't made of pure silver—you'd burn your hand off. Instead, we use plastics or materials rich in nonmetallic properties to keep the heat where it belongs: in the food.
- Bromine ($Br$): The only nonmetal that stays liquid at room temperature. It’s a nasty, reddish-brown liquid that smells terrible.
- Carbon ($C$): The MVP. It can be a soft, black graphite in your pencil or the hardest natural substance on Earth (diamond).
- Noble Gases: These guys are at the far right. Helium ($He$), Neon ($Ne$), Argon ($Ar$). They are so stable they barely react with anything. They are the "aristocrats" who don't want to mix with the commoners.
The "In-Betweeners": Making Sense of Metalloids
This is where things get really interesting for the tech world. Metalloids are the "Staircase" elements. They sit right on that jagged line between the metals and the nonmetals. Boron ($B$), Silicon ($Si$), Germanium ($Ge$), Arsenic ($As$), Antimony ($Sb$), Tellurium ($Te$), and Polonium ($Po$) make up this exclusive club.
👉 See also: How to Access Deleted iMessages Without Losing Your Mind
They are the ultimate fence-sitters. A metalloid might look like a metal—shiny and solid—but it behaves like a nonmetal in certain chemical reactions. Their most famous property? They are semiconductors.
Silicon is the poster child for this. It doesn't conduct electricity as well as Copper, but it doesn't block it as well as Glass. By "doping" Silicon (adding tiny amounts of other elements), we can control exactly how much electricity moves through it. This tiny bit of control is the entire reason we have microchips, computers, and the internet. Without the unique "maybe-yes, maybe-no" conductivity of metalloids, we'd still be using vacuum tubes the size of lightbulbs for basic math.
Comparing Metals Metalloids and Nonmetals Properties
If you're trying to spot these in the wild, you've gotta look at the physical and chemical "tells."
Metals usually have high melting points. Tungsten ($W$) is the king here, which is why it was used for years in lightbulb filaments. It can get incredibly hot without turning into a puddle. Nonmetals, on the other hand, have relatively low melting points. Many of them are already gases by the time they reach room temperature.
Chemical behavior is the real divider. Metals tend to form cations (positive ions) because they lose electrons. Nonmetals form anions (negative ions) because they gain them. When a metal and a nonmetal meet, they often form an ionic bond. Think Table Salt—Sodium (metal) gives an electron to Chlorine (nonmetal). They become $NaCl$. It's a match made in heaven. Metalloids? They usually prefer covalent bonding, where they share electrons rather than giving them up entirely.
📖 Related: Why the Bird Buddy Nature Station Is More Than Just a Smart Feeder
The Misconceptions We All Fall For
One of the biggest mistakes people make is thinking that "metal" equals "strong." Not always. Lead ($Pb$) is a metal, but it’s soft enough to scratch with your fingernail. Aluminum ($Al$) is light and relatively soft until it’s alloyed with other things. On the flip side, some nonmetals are incredibly "strong" in their own way. Diamond is a nonmetal, yet it can cut through almost any metal you put in front of it.
Another weird one is the idea that all metals are magnetic. Nope. Only a few—Iron ($Fe$), Nickel ($Ni$), and Cobalt ($Co$)—are truly ferromagnetic at room temperature. If you try to stick a magnet to a gold ring or an aluminum soda can, nothing happens.
Why Should You Care?
Understanding these distinctions is how we solve modern problems. When engineers are designing a new electric vehicle battery, they aren't just picking elements at random. They are looking at the specific metals metalloids and nonmetals properties to find the right balance of weight, energy density, and conductivity. Lithium ($Li$) is used because it's the lightest metal and has a massive "urge" to move its electrons, which creates the current that drives the car.
If we need something that can survive the vacuum of space, we look at metalloids and specific alloys that won't "outgas" or become brittle in the extreme cold. It’s all a giant puzzle.
Actionable Takeaways for Your Next Project (or Trivia Night)
- Identify by Luster: If it's dull and earthy, it’s probably a nonmetal. If it looks like a mirror, check the metal category.
- The "Snap" Test: If you try to bend a solid and it snaps or crumbles, you’re likely looking at a nonmetal or a very brittle metalloid. Metals will generally deform before they break.
- Check the Conductivity: If you're building a DIY circuit, stick to the left side of the periodic table for your wires. If you want to stop a short circuit, look to the right.
- Silicon is Key: If you're into electronics, remember that the "semi" in semiconductor refers to the metalloid property of being a "halfway" conductor.
Next time you see a periodic table, don't see it as a list of names to memorize. See it as a map. The left side is for building and powering, the right side is for life and insulation, and the middle staircase is the brain that makes our digital world possible.
To dive deeper, go grab a magnifying glass and look at the "lead" in your pencil (graphite) versus a piece of aluminum foil. Try to smudge the graphite—that's the nonmetal's layers sliding off because they aren't bonded like a metal. Then try to fold the foil. It stays together because of that metallic bond. That’s chemistry in your kitchen.