Imagine you’re back in the early 1800s. People have been mixing potions and melting metals for centuries, but honestly, nobody really knows why some stuff combines and other stuff just doesn't. Then comes John Dalton. He wasn't some flashy celebrity scientist; he was a Quaker schoolteacher from Manchester who spent his time obsessing over the weight of gases. His big breakthrough, the law of multiple proportions, basically changed everything about how we see the physical world. It’s the reason we know that $H_{2}O$ isn't just a random slurry of hydrogen and oxygen, but a specific, repeatable recipe.
It sounds technical. It sounds like something you'd ignore in a high school textbook. But it's actually the "secret code" of the universe.
The Law of Multiple Proportions: Breaking Down the Basics
Here is the gist of it. When two elements decide to hook up and form more than one compound, the masses of one element that combine with a fixed mass of the other are in the ratio of small whole numbers.
Think about carbon and oxygen. They’re like a couple that can have two different types of relationships. In one, you get Carbon Monoxide ($CO$). In the other, you get Carbon Dioxide ($CO_{2}$). If you take exactly 100 grams of carbon, you might find it reacts with 133 grams of oxygen to make the first one, or 266 grams to make the second.
Notice something? 266 is exactly double 133. It’s a 2:1 ratio.
It’s never a weird, messy number like 2.179 to 1. Nature likes simple math. Dalton realized this wasn't a coincidence. It meant that matter must be made of chunky, indivisible bits. Atoms. If you could have half an atom, those ratios would be all over the place. Since they stay as whole numbers, atoms have to stay whole too.
👉 See also: How to Log Off Gmail: The Simple Fixes for Your Privacy Panic
Why Does This Actually Matter?
Most people confuse this with the Law of Definite Proportions. That one says a specific compound—like pure water—always has the same ratio of elements. If you have water from a glacier or water from your tap, it's always roughly 11% hydrogen and 89% oxygen by mass.
But the law of multiple proportions is different because it compares two different compounds made of the same ingredients. It’s the difference between a recipe for bread and a recipe for pizza dough. Both use flour and water, but the ratios change the result entirely.
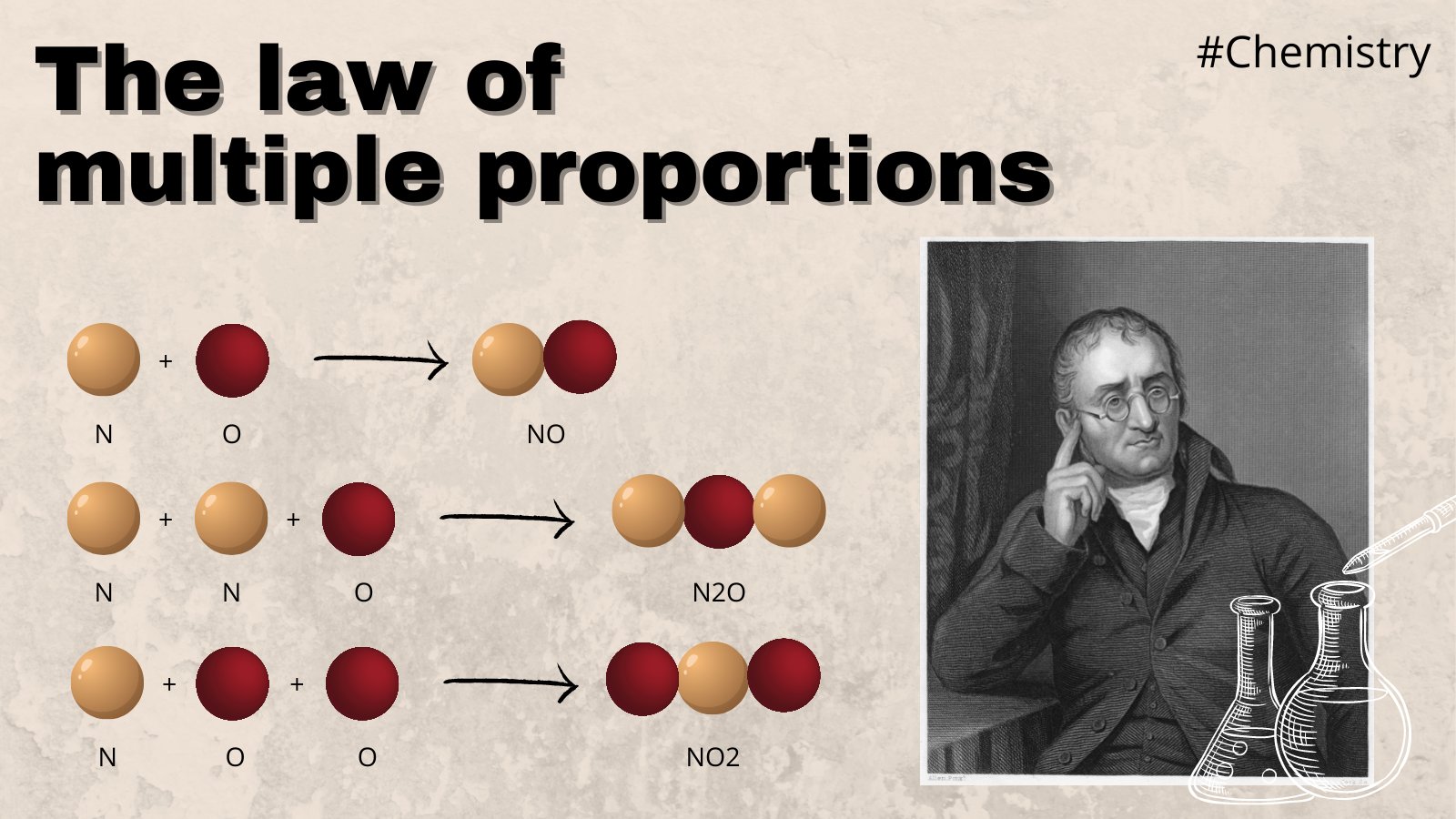
The Nitrogen Mystery
Nitrogen and oxygen are the ultimate examples here. They are like a chemistry experiment that keeps on giving. These two elements can form at least five different stable compounds.
- Nitrous oxide ($N_{2}O$) - Laughing gas.
- Nitric oxide ($NO$) - A signaling molecule in your body.
- Nitrogen dioxide ($NO_{2}$) - That gross brown smog you see over cities.
When scientists measured the oxygen that combined with a set amount of nitrogen across these different gases, they found ratios like 1:2:3:4:5. It’s almost too perfect. Dalton used these observations to argue that atoms weren't just a philosophical idea—they were a physical reality you could weigh.
How Dalton Fumbled (But Still Won)
Dalton wasn't perfect. He actually thought water was $HO$ instead of $H_{2}O$. He assumed nature was as simple as possible, so he figured if two elements only made one compound, it had to be a 1:1 ratio. Because his initial weights were slightly off due to the primitive tech of the 1800s, his specific data was a bit "meh," but his logic was bulletproof.
✨ Don't miss: Calculating Age From DOB: Why Your Math Is Probably Wrong
Other scientists like Jöns Jacob Berzelius eventually cleaned up the numbers. Berzelius was a stickler for detail. He spent years meticulously weighing elements and proved Dalton’s law was right across the board. This wasn't just some niche theory; it was the foundation of the Periodic Table. Without this law, we wouldn't have modern pharmaceuticals, materials science, or even the screen you're reading this on right now.
The "Whole Number" Rule and Modern Tech
You might think we’ve moved past this with our fancy particle accelerators and quantum physics. Nope. We still use the law of multiple proportions every single day in stoichiometry. If a chemical plant is making fertilizer, they rely on these exact ratios to ensure they don't waste millions of dollars in raw materials or, you know, blow up the factory.
There are some weird exceptions in the world of "non-stoichiometric compounds." These are mostly solid minerals where the crystal lattice has some holes in it, so the ratios look a bit "broken." But for the vast majority of chemistry, Dalton’s rules are still the law of the land.
Taking This Knowledge Further
Understanding this law isn't just about passing a chemistry quiz. It’s about recognizing the underlying order in a world that looks chaotic.
Identify the compounds: Next time you look at a label, see if you can spot elements that form multiple versions. Sulfur and oxygen are great ones to look for ($SO_{2}$ vs $SO_{3}$).
🔗 Read more: Installing a Push Button Start Kit: What You Need to Know Before Tearing Your Dash Apart
Calculate the ratios: If you're a student or a hobbyist, take the mass of Oxygen in $SO_{2}$ and $SO_{3}$ for a fixed mass of Sulfur. You’ll find that 3:2 ratio popping up instantly.
Check the sources: If you want to see Dalton's original messy sketches, the Science Museum Group has digitized many of his papers. Seeing his hand-drawn "circles" for atoms makes the law feel a lot more human and a lot less like a cold equation.
Apply it to Stoichiometry: Use these ratios to predict how much product you'll get in a reaction. It’s the fundamental skill for anyone going into lab work or chemical engineering.
Don't let the "old" science fool you. The law of multiple proportions is the reason we stopped guessing and started measuring. It’s the bridge between alchemy and the modern age.