You probably think you know water. It’s the stuff in your glass, the rain on your windshield, and roughly 60% of your own body. But when you ask a chemist is water a compound, the answer isn't just a simple yes. It’s a dive into how the universe actually glues itself together.
Most people mix up terms. Elements, mixtures, compounds—they all start to sound the same after a few years away from a high school classroom.
👉 See also: iPod Touch Fourth Gen Explained: Why the Chrome-Back Legend Still Matters
Water is a compound. Period. But understanding why it’s a compound and not just a weirdly intimate mixture of gases is where things get interesting. It’s about the transformation. When hydrogen and oxygen decide to hook up, they don't just hang out. They change. They become something entirely different from their original selves, and that's the secret sauce of chemistry.
The dead-simple reason water is a compound
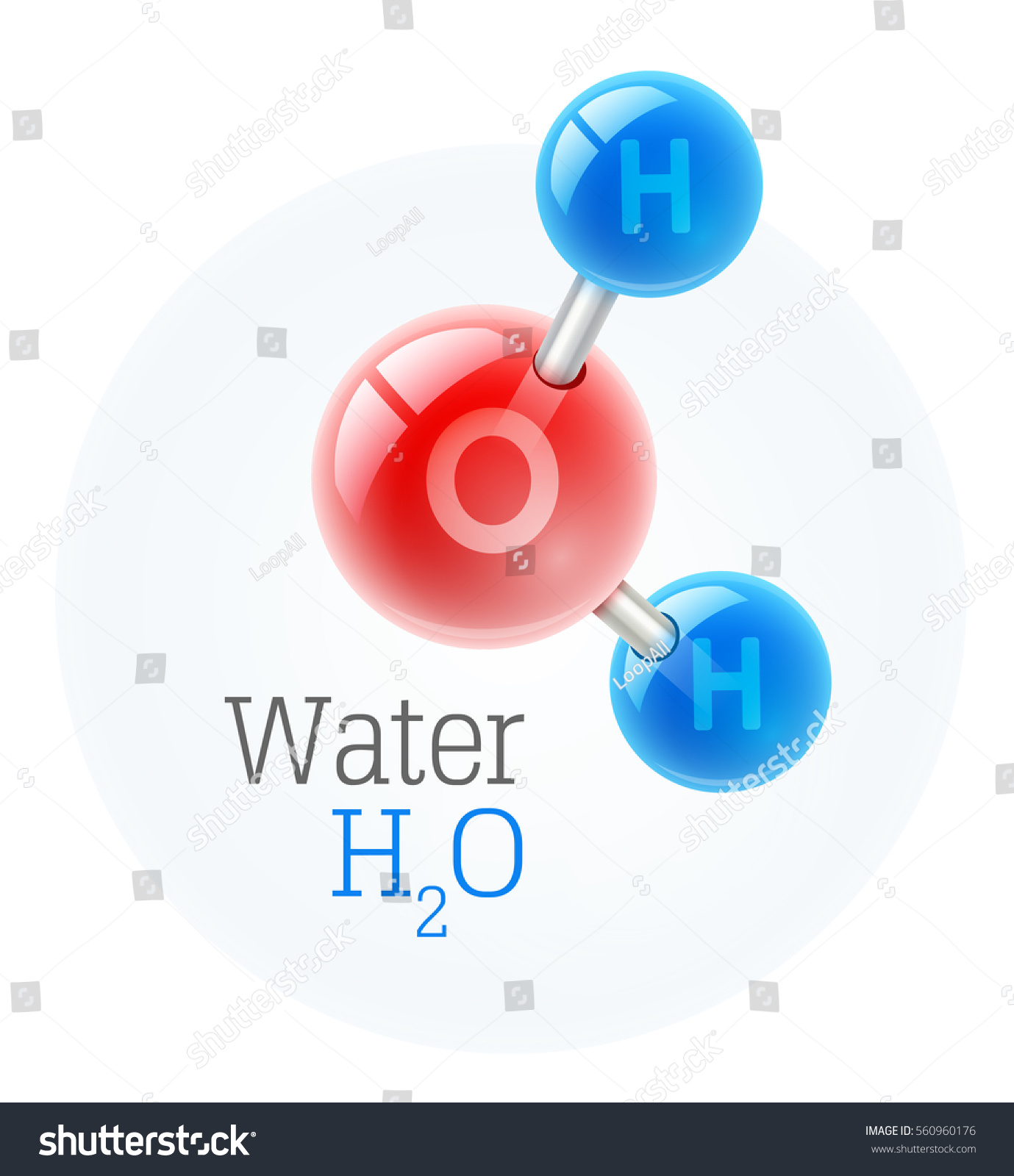
Let’s get the technicalities out of the way. A compound is a substance formed when two or more elements chemically bond together. In the case of water, we’re looking at two hydrogen atoms and one oxygen atom.
They aren't just floating near each other. They are locked in a covalent bond.
Think about it this way. Hydrogen is a highly flammable gas. Oxygen is a gas that literally fuels fire. If you just had a mixture of them, you’d have a high-pressure bomb waiting for a spark. But when they bond to form is water a compound, they create a liquid that we use to put out fires. That radical shift in properties is the definitive hallmark of a chemical compound.
It’s about fixed ratios. Always.
If you have two hydrogens and two oxygens, you don’t have extra-strength water. You have hydrogen peroxide ($H_2O_2$), which will bleach your hair or sting your cuts. Compounds are picky. They require a specific "recipe" that never changes, regardless of whether the water came from a glacier in Antarctica or a tap in New Jersey.
Why it's not a mixture (and why that matters)
People often confuse compounds with mixtures. It's an easy mistake.
A mixture is like a salad. You throw in some lettuce, some tomatoes, and some croutons. You can pick the croutons out if you’re keto. The lettuce is still lettuce. In a mixture, the individual components keep their original identities.
Water doesn't work like that.
You can't "sift" the oxygen out of a water molecule. You can't use a filter to separate the hydrogen. To get those elements back, you have to perform a chemical reaction, like electrolysis. By running an electric current through the liquid, you can force those bonds to snap, releasing the gases back into the air.
Honestly, the way the atoms share electrons is almost poetic. In a water molecule, the oxygen atom is a bit of an electron hog. It pulls the negative charge toward itself, creating a polar molecule. This "polarity" is why water is the "universal solvent." It’s why salt dissolves in your pasta water and why your blood can carry minerals through your veins.
The geometry of a drop
If you could zoom in—way in—you’d see that water isn't a straight line. It's bent. The atoms sit at an angle of about 104.5 degrees.
This v-shape is why ice floats. Most things get denser when they freeze, but water is a rebel. Because of its compound structure, it forms a crystalline lattice when it gets cold, pushing the molecules further apart. If water weren't a compound with this specific geometry, lakes would freeze from the bottom up, killing all aquatic life, and Earth would be a very different, very dead rock.
Common misconceptions about H2O
I’ve heard people argue that "pure" water doesn't exist in nature, so it must be a mixture.
They're half right, but for the wrong reasons.
While it's true that the water in your river or even your "purified" bottled water contains dissolved minerals (like magnesium or calcium) and gases, the water itself remains a compound. The stuff in the bottle is a mixture of the compound $H_2O$ and other various elements.
- Is tap water a compound? No, it's a homogeneous mixture.
- Is the $H_2O$ molecule inside that tap water a compound? Yes, absolutely.
- Is steam a compound? Yes, it's just the compound in a gaseous state.
Distinction is key here. Chemists refer to "pure water" as a substance consisting only of $H_2O$ molecules. In the real world, we rarely encounter it because water is so good at dissolving things that it picks up "hitchhikers" wherever it goes.
The role of energy in forming the bond
You can't just shove hydrogen and oxygen together and hope for the best. They need a "push" to bond.
This usually involves a spark or heat. When that happens, the reaction is exothermic—it releases a massive amount of energy. This is exactly what powered the Space Shuttle's main engines. They used liquid hydrogen and liquid oxygen. When they reacted to form the compound water, the explosion of energy was enough to lift tons of metal into orbit.
It’s wild to think that the byproduct of rocket science is the same stuff you use to boil an egg.
Beyond the basics: Isotopes and Heavy Water
Is water always $H_2O$? Well, mostly.
There's this stuff called "Heavy Water" or deuterium oxide ($D_2O$). Deuterium is a "heavy" isotope of hydrogen that has a neutron (normal hydrogen doesn't have any). It’s still a compound. It still behaves mostly like water, but it’s about 11% denser.
Nuclear reactors use it to slow down neutrons. While it looks and tastes like normal water, if you drank only heavy water for several days, it would eventually mess up your internal chemistry because those slightly heavier bonds change the speed of chemical reactions in your body.
How to identify a compound in the wild
If you're ever unsure if something is a compound or just a mixture, ask yourself these three things:
- Does it have a chemical formula? If you can write it down like $NaCl$ or $H_2O$ or $CO_2$, it’s a compound.
- Does it require a reaction to separate? If you can't separate it with a sieve, a magnet, or a centrifuge, you’re likely looking at a compound.
- Did the properties change? If the "ingredients" were totally different from the "final product," a chemical change occurred.
Water hits every single one of these marks.
Practical insights for the curious mind
Knowing that water is a compound isn't just for passing chemistry quizzes. It changes how you look at the world. It helps you understand why "dehydrated" foods work or why electrolysis is a potential path for clean energy (hydrogen fuel cells).
Actionable Next Steps:
- Check your labels: Look at "mineral water" bottles. You’ll see the compound $H_2O$ listed alongside elements like Sodium and Magnesium. Note that the water is the solvent, and the others are the solutes.
- Visualize the bond: Next time you see a raindrop on a window, remember the "bent" shape of the molecule. That specific angle is the reason the drop sticks to the glass (adhesion) and to itself (cohesion).
- Experiment with separation: If you're feeling adventurous, look up a safe "pencil and battery" electrolysis experiment. You can actually see the water compound being ripped apart into oxygen and hydrogen bubbles right in front of your eyes.
- Think about filtration: Understand that a carbon filter (like a Brita) removes mixtures—like chlorine or lead—but it never changes the water compound itself.
Water is the most studied substance on the planet, yet it still manages to surprise us. It's the only substance that naturally exists as a solid, liquid, and gas at the temperatures found on Earth. All of that weirdness, all that life-giving potential, stems from the simple fact that it is a perfectly balanced chemical compound.