You’re standing in a kitchen. On the counter sits a glass of water and a bottle of nail polish remover. If you leave them both open overnight, the polish remover is gone by morning. The water? It’s barely budged. This isn't magic. It's vapor pressure in action, and honestly, understanding how is high to low vapor pressure ranked is basically just a game of figuring out how "sticky" molecules are to one another.
Vapor pressure is that invisible force. Specifically, it’s the pressure exerted by a vapor in thermodynamic equilibrium with its condensed phases at a given temperature in a closed system. Sounds fancy, right? It’s really just a measure of how badly a liquid wants to turn into a gas. When we rank substances from high to low vapor pressure, we are essentially ranking them from the most "flighty" or volatile to the most "stubborn" or stable.
The Secret Ranking Factor: Intermolecular Forces
If you want to know why some things evaporate while others sit there for years, you have to look at Intermolecular Forces (IMFs). These are the "glues" holding molecules together.
When ranking how is high to low vapor pressure ranked, the rule is inverse. The stronger the glue, the lower the vapor pressure. It makes sense if you think about it. If molecules are hugging each other tightly, they aren't going to just float away into the air.
There are three main types of "glue" we look at in chemistry:
- London Dispersion Forces: These are the weakest. Every molecule has them, but in non-polar stuff like methane or gasoline, it’s all they’ve got. Since the bond is weak, these substances have sky-high vapor pressure.
- Dipole-Dipole Interactions: These happen in polar molecules. Think of them like magnets. They’re stronger than dispersion forces, so these substances usually sit in the middle of our ranking.
- Hydrogen Bonding: This is the heavyweight champion. Water is the classic example. Because water molecules love each other so much (via hydrogen bonds), they don't want to leave the liquid phase. This gives water a surprisingly low vapor pressure compared to other small molecules.
Ranking in Action: From Volatile to Solid
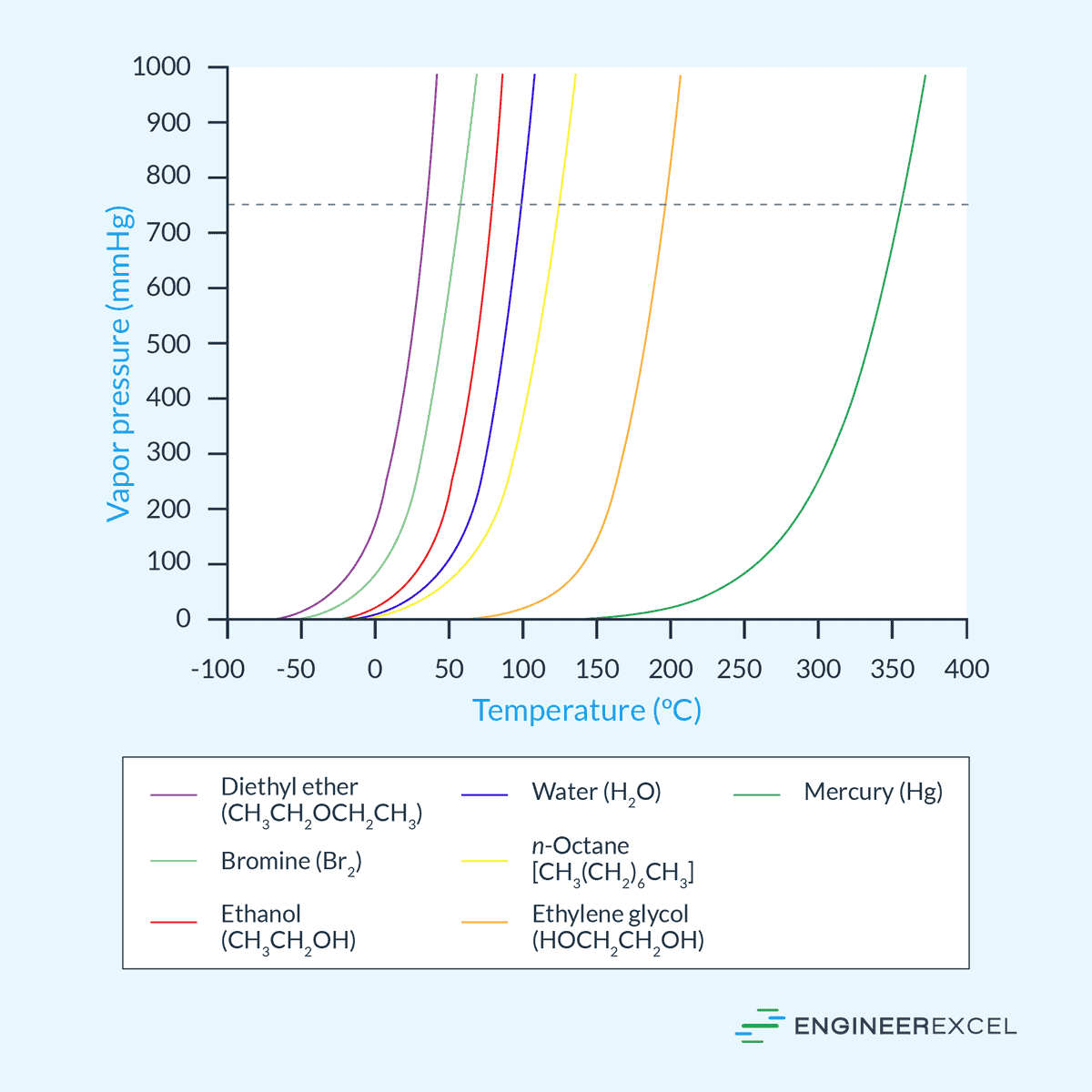
Let’s look at some real-world examples. If we were to create a list ranking substances from high vapor pressure to low vapor pressure at room temperature (around 25°C), it would look something like this:
✨ Don't miss: Is Duo Dead? The Truth About Google’s Messy App Mergers
Diethyl Ether takes the top spot. It’s incredibly volatile. You open a bottle, and the smell hits you instantly because the molecules are practically sprinting into the air. Its vapor pressure is roughly 530 mmHg.
Next, you've got Acetone. This is your standard nail polish remover. It has a high vapor pressure (about 230 mmHg), but it's not quite as "eager" as ether.
Then we hit Ethanol. Alcohol has hydrogen bonding, but it’s not as extensive as water's. Its vapor pressure sits around 45 mmHg.
Water is the big surprise for many. Even though it’s a tiny molecule, its vapor pressure is only about 24 mmHg at room temp. Those hydrogen bonds are doing some heavy lifting.
Finally, at the bottom of the "high to low" ranking, you have things like Mercury or Ionic Liquids. Mercury is a liquid metal with a vapor pressure so low (0.0017 mmHg) that it barely evaporates at all under normal conditions.
🔗 Read more: Why the Apple Store Cumberland Mall Atlanta is Still the Best Spot for a Quick Fix
The Temperature Variable
You can’t talk about how vapor pressure is ranked without mentioning temperature. It’s the great equalizer. As you heat things up, you’re basically injecting energy into the molecules. You’re giving them the "kick" they need to break free from their neighbors.
Have you ever noticed how a puddle disappears way faster on a 90-degree day than a 50-degree day? That’s because vapor pressure increases exponentially with temperature. When the vapor pressure of a liquid finally matches the atmospheric pressure around it, you’ve reached the boiling point.
This is why water boils faster in Denver than in Miami. In Denver, the atmospheric pressure is lower. The water doesn’t need as much "push" (vapor pressure) to match the air, so it starts boiling at a lower temperature.
The Clausius-Clapeyron Connection
If you’re looking for the math behind the ranking, you’ll eventually run into the Clausius-Clapeyron equation. It looks scary, but it’s just a way to predict how the vapor pressure will change as temperature moves.
$$\ln(P_1/P_2) = \frac{\Delta H_{vap}}{R} \left(\frac{1}{T_2} - \frac{1}{T_1}\right)$$
💡 You might also like: Why Doppler Radar Overland Park KS Data Isn't Always What You See on Your Phone
In this formula, $\Delta H_{vap}$ is the enthalpy of vaporization—the energy required to vaporize one mole of the substance. Substances with a high $\Delta H_{vap}$ have very low vapor pressures because it takes a massive amount of "bribe money" (energy) to get those molecules to move.
Why Does This Ranking Matter?
It’s not just for lab coats and textbooks. Understanding how is high to low vapor pressure ranked is vital for a bunch of industries.
In the automotive world, gasoline has to be "tuned" for the season. Winter-blend gasoline has a higher vapor pressure. Why? Because when it’s freezing outside, gas needs to evaporate easily enough to start your cold engine. If they used summer-blend gas (lower vapor pressure) in the winter, your car might struggle to turn over.
In the world of perfumes and colognes, "top notes" are just ingredients with high vapor pressure. They hit your nose first because they evaporate the fastest. The "base notes" like musk or sandalwood have very low vapor pressure, which is why you can still smell them on your skin hours later.
Practical Tips for Working with Vapor Pressure
- Store Volatiles Cold: If you have chemicals or solvents with high vapor pressure (like gasoline or certain paints), keep them in a cool place. Lowering the temperature drops the vapor pressure, reducing the risk of pressure buildup or fumes.
- Check Sealing: High vapor pressure liquids will find any gap in a seal. Use PTFE tape or high-quality gaskets for containers holding alcohols or ethers.
- Safety First: Remember that high vapor pressure often goes hand-in-hand with flammability. The more vapor in the air, the easier it is for a stray spark to cause a problem.
- Altitude Adjustments: If you are canning food or brewing beer at high altitudes, remember that your liquids will reach their "ranking" of vapor pressure parity much sooner than at sea level.
Understanding the hierarchy of vapor pressure is really about understanding the internal tug-of-war within matter. By looking at the molecular structure and the temperature, you can accurately predict and rank how any substance will behave when exposed to the open air. Low vapor pressure means "staying put," while high vapor pressure means "moving out."
Moving forward, whenever you're dealing with a liquid, check its Safety Data Sheet (SDS) for the vapor pressure value. If it's over 100 mmHg at room temperature, treat it as highly volatile. If it's under 10 mmHg, it's relatively stable. This simple check can save you from unexpected fumes or lost product in almost any DIY or professional setting.