Chemistry students often hit a wall when they first see log functions. It’s intimidating. You’re staring at a lab report or a midterm exam, and you have the $pKa$ value, but the formula you need to use actually requires the acid dissociation constant, or $Ka$. So, how do you get Ka from pKa without losing your mind?
It’s actually a straightforward inverse calculation. If you can use a calculator, you can do this in about four seconds.
Basically, $pKa$ is just a shorthand. Scientists got tired of writing tiny decimals with ten zeros after the decimal point, so they used a negative logarithm to make the numbers look like normal, human integers. To go backward and find the $Ka$, you just need to undo that logarithm.
The Mathematical Escape Room
To understand the "how," you have to understand the "what." In chemistry, the "$p$" in $pKa$ (or $pH$ or $pOH$) literally stands for "power" or "potential," but mathematically, it’s an operator. It means "$-\log_{10}$."
When someone asks how do you get Ka from pKa, they are asking for the antilog.
The formula is:
$$Ka = 10^{-pKa}$$
Think of it like a mirror. If $pKa = -\log(Ka)$, then to get $Ka$ back, you raise 10 to the power of the negative $pKa$.
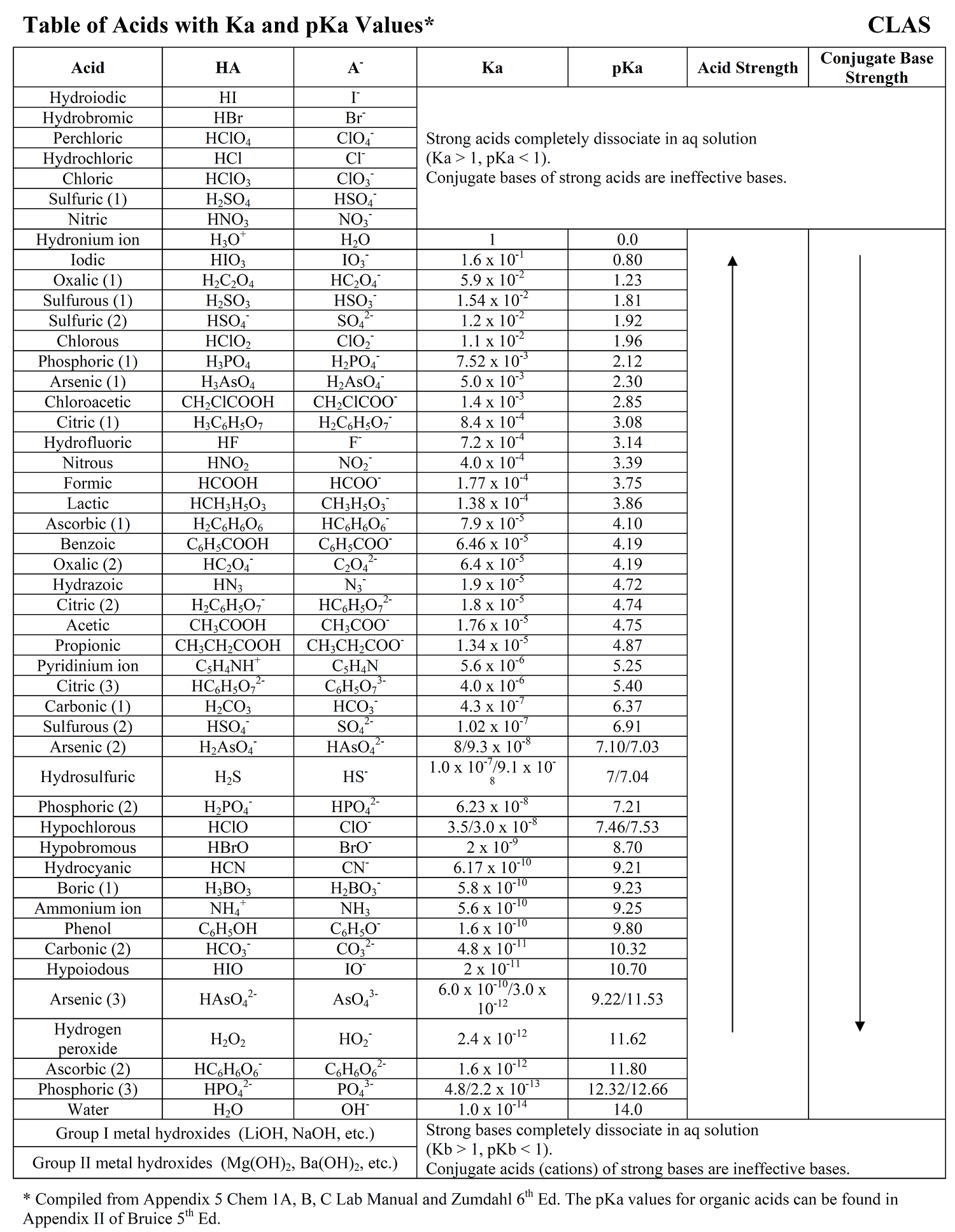
Let’s say you have a $pKa$ of 4.76 (which is the $pKa$ for acetic acid, the stuff in vinegar). To find the $Ka$, you just plug it in: $10^{-4.76}$. On most scientific calculators, you’ll hit the "10^x" button or the "Shift/2nd" then "log" button.
The result is $1.74 \times 10^{-5}$.
It’s a tiny number. That’s why we use $pKa$ in the first place. Imagine trying to talk about $0.0000174$ in a fast-paced lecture. It’s annoying. 4.76 is just easier to say.
📖 Related: Why Every Math Student Eventually Needs a Factor Completely Calculator with Steps
Why This Conversion Actually Matters
You might wonder why we even bother switching back and forth. Honestly, it’s about context.
If you are working with the Henderson-Hasselbalch equation to design a buffer for a biological study, you’re usually working with $pKa$. It fits the equation perfectly. But if you are trying to calculate the exact concentration of hydrogen ions $[H^+]$ in a solution using an ICE table (Initial, Change, Equilibrium), you need the $Ka$.
The $Ka$ tells you exactly how much an acid "wants" to fall apart in water.
A high $Ka$ means a strong acid. It’s aggressive. It dissociates completely.
A low $Ka$ (which means a high $pKa$) means a weak acid. It’s clingy. It holds onto its protons.
Take hydrochloric acid ($HCl$). Its $pKa$ is somewhere around -6 or -7. If you do the math—$10^{-(-7)}$—you get a $Ka$ of $10,000,000$. That is a massive number. It tells you that $HCl$ is basically an explosion of ions the second it touches water.
Contrast that with hydrocyanic acid ($HCN$). It has a $pKa$ of about 9.21.
$$Ka = 10^{-9.21} \approx 6.2 \times 10^{-10}$$
That is incredibly small. It barely dissociates at all.
Common Pitfalls: The Negative Sign
The biggest mistake people make? Forgetting the negative sign in the exponent.
If you type $10^{4.76}$ instead of $10^{-4.76}$, you get a huge number like 57,543. If you’re dealing with a weak acid like vinegar and your $Ka$ comes out to fifty-seven thousand, something is wrong. Your beaker would probably be melting.
Always check for "reasonableness."
- If your $pKa$ is positive, your $Ka$ must be a very small decimal (scientific notation with a negative exponent).
- If your $pKa$ is negative, your $Ka$ will be a large number greater than 1.
Real-World Applications in Lab Work
In pharmacology, the $pKa$ of a drug determines how well it’s absorbed in your body. Most drugs are either weak acids or weak bases.
If a drug has a certain $pKa$ and it enters your stomach (which has a very low $pH$), the ratio of the ionized form to the non-ionized form changes. This ratio is governed by the $Ka$. If a researcher needs to know the exact percentage of a drug that will cross a cell membrane, they have to convert that $pKa$ back to $Ka$ to run the equilibrium constants.
💡 You might also like: Why the Apple Store Country Club Plaza Kansas City is Still a Tech Hub Essential
It’s not just academic torture; it’s how we figure out dosages for ibuprofen or aspirin.
Moving Toward Mastery
Once you’ve mastered how do you get Ka from pKa, the next step is usually handling bases. It’s the same logic, just different letters. $pKb$ becomes $Kb$ using the same $10^{-x}$ trick.
Just remember that at $25^\circ C$, $pKa + pKb = 14$. This is a constant. If you know one, you know them all. It’s like a puzzle where all the pieces are linked by powers of ten.
To get started with your own calculations:
- Identify the pKa value from your reference table or problem statement.
- Toggle your calculator to scientific mode.
- Enter the value as a negative exponent of 10.
- Verify the magnitude—weak acids should always result in small $Ka$ values (e.g., $10^{-4}$ to $10^{-10}$).
- Apply the Ka to your equilibrium expression or ICE table to find the molarity of your ions.
By converting these logarithmic values back into standard constants, you gain a clearer picture of the chemical equilibrium at play, allowing for precise control over pH levels in everything from industrial fermentation to skincare formulation.