We know so little about the first week of life. It’s wild, honestly. While we’ve mapped the human genome and sent rovers to Mars, the moment an embryo actually attaches to the uterine wall—a process called 3D human embryo implantation—has remained a total mystery. Scientists literally call it the "black box" of human development.
You can’t just watch it happen in a person. Ethical constraints and the sheer microscopic scale of the event make direct observation impossible. But things are changing fast. Thanks to breakthroughs in synthetic biology and advanced imaging, researchers are finally building 3D models that mimic the complex "crosstalk" between an embryo and the mother.
Why 3D Human Embryo Implantation Models Change Everything
For decades, we relied on mice. But humans aren't big mice. Our embryos implant differently, invading the uterine lining much more aggressively than rodents do.
If you look at the work coming out of places like the University of Cambridge or the Hubrecht Institute, you’ll see they aren't just using flat petri dishes anymore. Traditional 2D cultures fail because cells behave differently when they can’t grow "up" and "around" each other. In a flat dish, a cell only has neighbors on its sides. In 3D, it experiences pressure and signals from every direction. This mechanical tension is what actually triggers the embryo to start organizing itself into a body.
The Role of Organoids and "Blastoids"
Researchers like Nicolas Rivron have pioneered the creation of "blastoids." These are structures made from stem cells that look and act like early human embryos but can't actually grow into a person. They’re models. Basically, they allow scientists to test how an embryo reacts to the uterine environment without using actual human embryos from IVF clinics.
By placing these blastoids into 3D-printed scaffolds or "organ-on-a-chip" devices that mimic the uterine lining (the endometrium), scientists can finally see the handshake. It’s a chemical conversation. The embryo sends a signal saying, "I’m here," and the mother’s tissue responds by remodeling itself to wrap around the newcomer.
When this conversation fails, pregnancy doesn't happen.
📖 Related: Why PMS Food Cravings Are So Intense and What You Can Actually Do About Them
The Mechanics of the "Handshake"
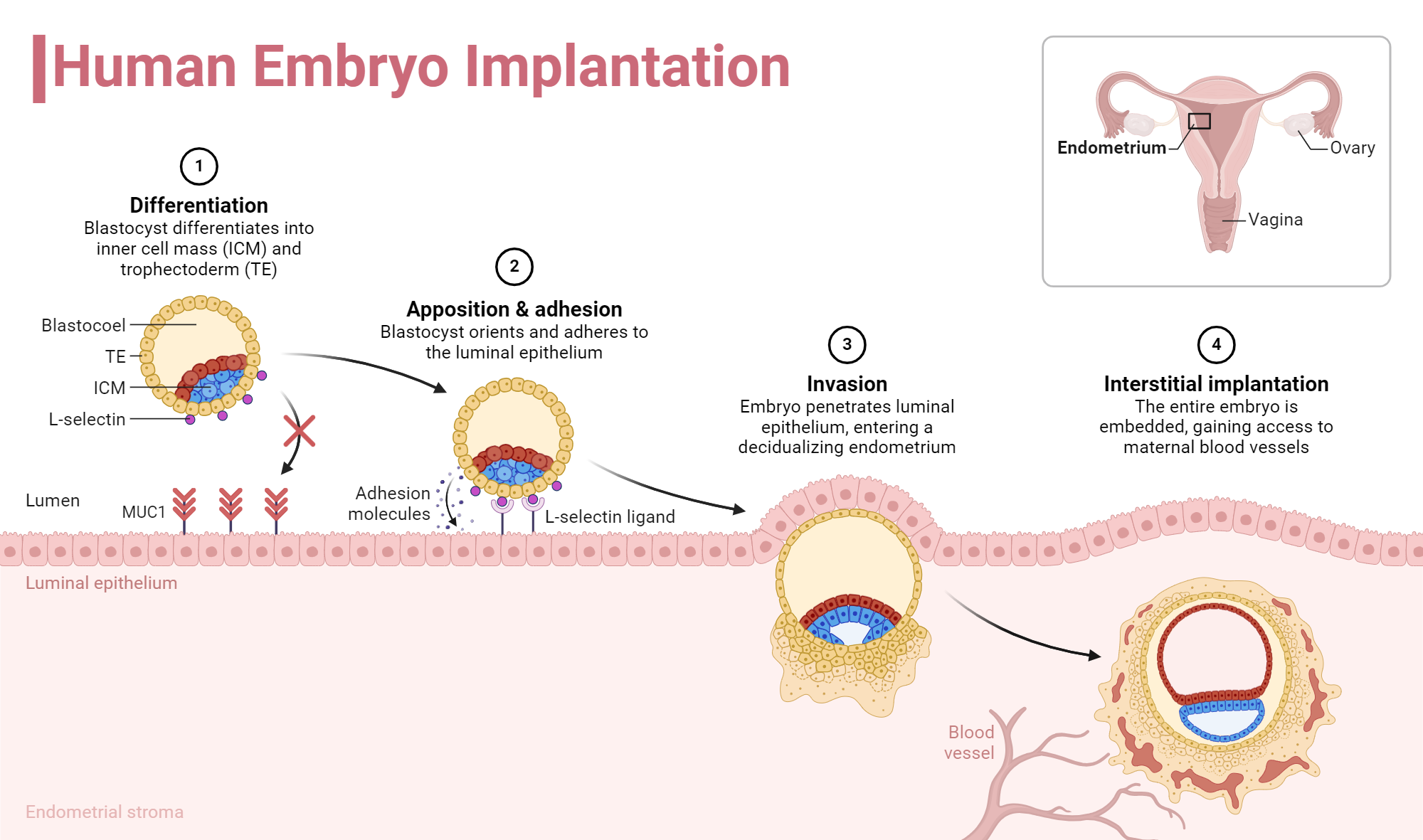
The process isn't just a physical sticking-together. It's an invasion. Around day six or seven after fertilization, the embryo—now a blastocyst—must hatch from its protective shell.
It then reaches out with specialized cells called trophoblasts. In a 3D human embryo implantation scenario, these cells burrow deep. They don't just sit on top; they remodel the mother's blood vessels. This is where it gets sketchy for the health of the pregnancy. If they don't burrow deep enough, you get preeclampsia later on. If they burrow too deep, you get placenta accreta.
Understanding the "depth" of this invasion is only possible in 3D models. You need that vertical dimension to measure how far those cells are traveling.
- Apposition: The embryo finds a spot and stays still.
- Adhesion: Stronger proteins, like integrins, lock the embryo in place.
- Invasion: The embryo literally eats into the uterine tissue to find blood.
It's intense.
Breaking Down the "Black Box" Statistics
Roughly 75% of pregnancy losses happen because of implantation failure. That is a staggering number. Most women don't even know they were pregnant; they just think their period was a few days late.
In the IVF world, we’ve gotten really good at making high-quality embryos. We can screen them for chromosomal issues (PGT-A testing). We can grade them based on how pretty they look under a microscope. Yet, even a "perfect" embryo often fails to stick. Why? Because the uterine environment is half the battle.
👉 See also: 100 percent power of will: Why Most People Fail to Find It
Recent studies using 3D bio-printing have shown that the "stiffness" of the uterine lining matters. If the tissue is too scarred or too soft, the embryo’s 3D structure collapses. It can't generate the force needed to break through the surface.
New Tech: From 2D to 3D Scaffolds
We are moving away from simple cell lines. Modern labs are using decellularized uterine tissue. They take actual human uterine tissue, strip away the cells, and leave behind the "skeleton" of collagen and proteins. Then, they seed this skeleton with fresh cells from a patient.
This creates a personalized 3D map.
You’ve gotta realize how big this is for personalized medicine. Imagine a woman who has had three failed IVF transfers. Doctors could potentially take a biopsy of her lining, grow a 3D version of it in a lab, and then test "mock" implantations to see exactly why her specific tissue is rejecting the embryos. We aren't quite there yet for daily clinical use, but the proof-of-concept is solid.
The Ethical Tightrope
Of course, this is controversial. The "14-day rule" has long been the gold standard for embryo research—stating that no human embryo can be cultured past the point where the nervous system starts to form.
However, because these 3D models are often "synthetic" (derived from stem cells, not sperm and egg), the legal definitions are getting blurry. The International Society for Stem Cell Research (ISSCR) recently updated their guidelines to allow for more flexibility, provided there is a strong scientific justification.
✨ Don't miss: Children’s Hospital London Ontario: What Every Parent Actually Needs to Know
What This Means for the Future of IVF
Right now, IVF is a bit of a numbers game. You put the embryo in and hope for the best.
By mastering the science of 3D human embryo implantation, we move toward "precision implantation." We might eventually develop "receptivity primers"—specific cocktails of growth factors tailored to a woman’s unique 3D uterine architecture.
It’s also helping us understand endometriosis. We’re finding that in women with endo, the 3D structure of the uterine lining is fundamentally disorganized. The cells are "noisy," making it hard for the embryo to hear the signals it needs to survive.
Actionable Insights for Patients and Researchers
If you are currently navigating fertility struggles or are simply curious about the cutting edge of reproductive biology, here is how to apply this knowledge:
For Patients:
- Ask about "Receptivity" Testing: While 3D modeling is still largely in the research phase, tests like the ERA (Endometrial Receptivity Array) are the first generation of tools trying to solve the "timing" part of the implantation puzzle.
- Focus on Inflammation: Since we know implantation is an active tissue-remodeling event, reducing systemic inflammation through diet and lifestyle can theoretically make the uterine "soil" more hospitable for the 3D invasion process.
- Advocate for Advanced Imaging: If you've had multiple failures, ask your specialist about 3D ultrasounds to look for structural abnormalities in the uterine wall that might interfere with the physical mechanics of attachment.
For Researchers and Clinicians:
- Transition to Hydrogels: If you are still using plastic surfaces for culturing, switching to stiffness-matched hydrogels can significantly improve the physiological relevance of your data.
- Incorporate Flow: Remember that the uterus is not a static environment. Integrating microfluidics to simulate the flow of uterine secretions adds a necessary layer of realism to any implantation model.
The move from seeing an embryo as a "dot on a screen" to understanding it as a dynamic, 3D entity that actively negotiates its survival is the biggest shift in reproductive science in forty years. We are finally turning the lights on in the black box.