Ever watched a campfire and wondered about the invisible math keeping you warm? Honestly, most people just see flames and feel the heat. But if you’re a chem student or an engineer, those flames represent a very specific, measurable energy release. We’re talking about the formula for enthalpy of combustion, a concept that sounds dry until you realize it’s the reason your car moves and your stove boils water.
It’s about energy. Specifically, the heat energy released when one mole of a substance undergoes complete combustion with oxygen under standard conditions. You've probably seen the symbol $\Delta H_c^\circ$. That little circle? It means standard conditions—usually $298\text{ K}$ and $100\text{ kPa}$.
If you get the math wrong here, your rockets don't launch and your engines melt. It’s that serious.
The Core Formula for Enthalpy of Combustion
Let's cut to the chase. You usually aren't measuring enthalpy directly; you're measuring a temperature change in a surroundings, like water in a calorimeter. This is where most people trip up. They confuse the heat change of the water ($q$) with the enthalpy change of the fuel.
The basic relationship starts with:
$$q = mc\Delta T$$
In this setup, $m$ is the mass of the substance being heated (usually water), $c$ is the specific heat capacity (for water, it’s about $4.18\text{ J/g}\cdot\text{K}$), and $\Delta T$ is the change in temperature.
But $q$ isn't your answer. To find the actual formula for enthalpy of combustion, you have to relate that heat to the amount of fuel you actually burned. That's where $n$ comes in—the number of moles.
$$\Delta H_c = \frac{-q}{n}$$
Why the negative sign? Because combustion is exothermic. It gives off heat. If the water gets hotter, the fuel is losing energy. If you forget that negative sign on a lab report, you’re basically claiming that your fire sucked heat out of the room and turned into ice. Physics doesn't work that way.
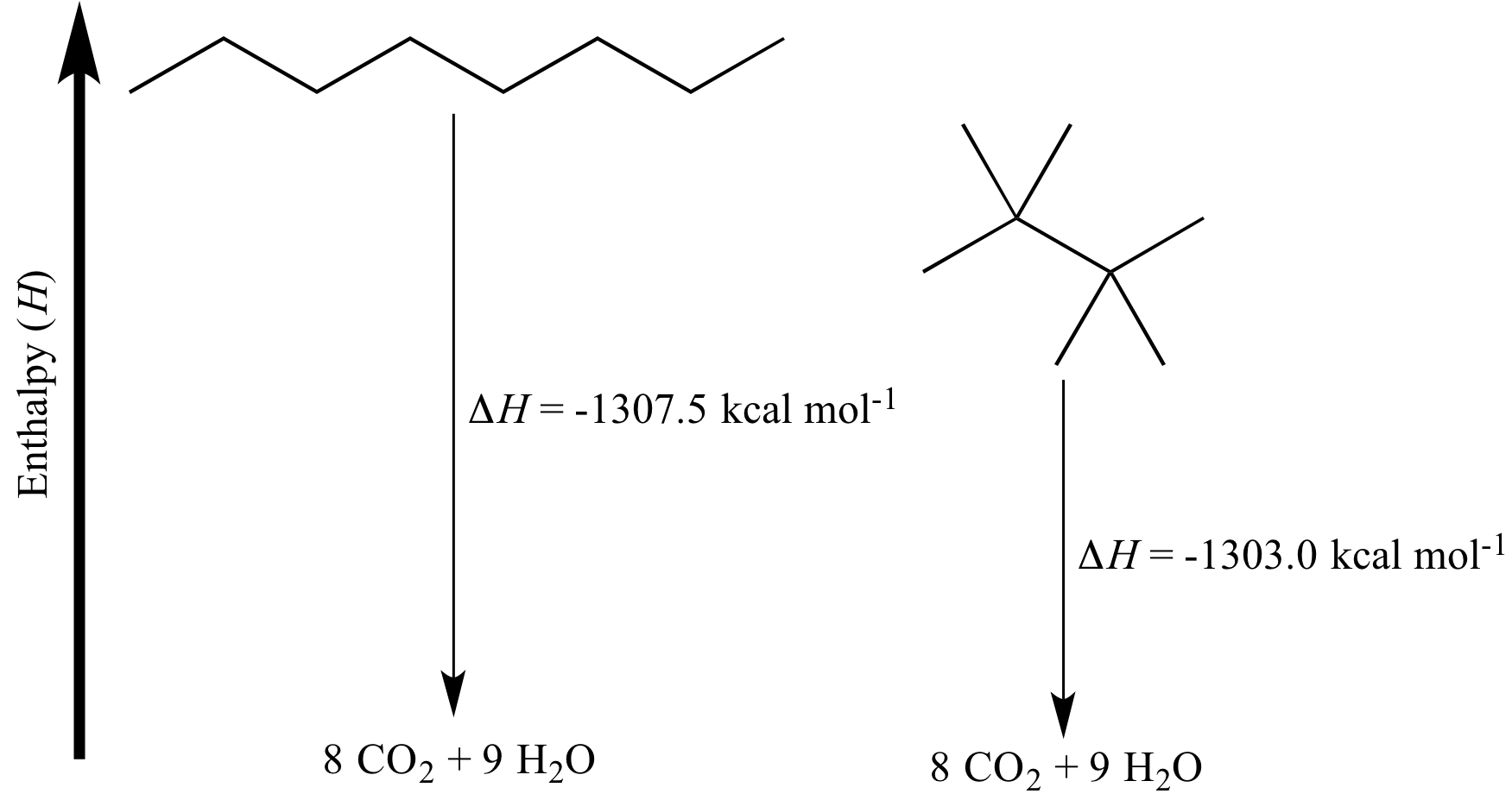
Why Complete Combustion is a Lie (Mostly)
In a perfect world, every carbon atom in your fuel finds two oxygen atoms and becomes $\text{CO}_2$. Every hydrogen atom finds an oxygen and becomes $\text{H}_2\text{O}$.
✨ Don't miss: Why Every Picture of a Wavelength You’ve Seen Is Probably Lying to You
Real life is messier.
If you’re burning a candle or a spirit lamp in a high school lab, you’re going to see yellow flames and black soot. That soot is pure carbon. It’s the physical manifestation of incomplete combustion. When you have incomplete combustion, you aren't releasing the full potential energy of the fuel. You’re making carbon monoxide ($\text{CO}$) or just plain old soot instead of $\text{CO}_2$.
This is why your experimental value for the formula for enthalpy of combustion is almost always lower than the data book value. You’ve got heat escaping to the air. You’ve got the copper can soaking up energy. You’ve got incomplete combustion. It’s a miracle we get close results at all, frankly.
Hess’s Law: The Secret Backdoor
Sometimes you can't just set something on fire and measure it. Maybe the reaction is too dangerous, or it’s too slow, or it just won't burn cleanly. This is where Germain Hess, a Russian chemist with a very practical mind, comes in.
Hess’s Law basically says that the total enthalpy change for a reaction is the same regardless of the route taken. It’s like hiking a mountain; the change in your altitude is the same whether you take the steep path or the long, winding trail.
For combustion, we use Enthalpies of Formation ($\Delta H_f^\circ$) to find the enthalpy of combustion. The formula looks like this:
$$\Delta H_{reaction}^\circ = \sum \Delta H_f^\circ(\text{products}) - \sum \Delta H_f^\circ(\text{reactants})$$
If you’re looking at the combustion of methane ($\text{CH}_4$):
🔗 Read more: Why the 40 mm standard range sponge round is still the go-to for crowd control
- Write the balanced equation: $\text{CH}_4 + 2\text{O}_2 \rightarrow \text{CO}_2 + 2\text{H}_2\text{O}$
- Look up the formation values for $\text{CO}_2$ and $\text{H}_2\text{O}$.
- Subtract the formation value of $\text{CH}_4$.
- Note: The formation value of $\text{O}_2$ is zero because it’s an element in its standard state.
Bond Enthalpies: The Lego Method
Think of molecules as Legos. To burn them, you have to snap the old blocks apart (which takes energy) and snap them back together in new ways (which releases energy).
Breaking bonds = Endothermic (requires energy, $+$)
Making bonds = Exothermic (releases energy, $-$)
The formula for enthalpy of combustion using bond enthalpies is:
$$\Delta H = \sum(\text{bond enthalpies of reactants}) - \sum(\text{bond enthalpies of products})$$
Wait. Did you notice the order flipped? In the formation formula, it was Products minus Reactants. In bond enthalpies, it’s Reactants minus Products. This is a classic "gotcha" on chemistry exams.
The reason is simple: you have to input energy to break the reactant bonds. So those are positive values. You get energy back when product bonds form. So those are subtracted.
Real-World Nuance: The Water Vapor Problem
Here is something even some college students forget: the state of the water produced.
When you burn hydrogen or a hydrocarbon, the water usually leaves the flame as steam (gas). However, "standard" enthalpy of combustion defines the final state of water as a liquid ($25^\circ\text{C}$).
There is a huge energy difference between water as a gas and water as a liquid. If your engine exhausts water vapor, you aren't capturing the "latent heat of vaporization." This leads to two different values you might see in industry:
- Higher Heating Value (HHV): Assumes the water is condensed back to liquid.
- Lower Heating Value (LHV): Assumes the water stays as vapor.
In most real-world combustion engines, you're looking at the LHV because we don't usually condense exhaust inside the engine. If you use the wrong one in your calculations, your efficiency ratings will be off by about $10%$.
Practical Steps for Accurate Calculations
If you're actually doing this in a lab or for a project, stop relying on "perfect" numbers. Here is how to actually handle the formula for enthalpy of combustion like a pro.
1. Calibrate your equipment
Don't just assume water takes $4.18\text{ J/g}\cdot\text{K}$. Your container (the calorimeter) absorbs heat too. Run a "blank" or a known substance first to find the "calorimeter constant." This is basically a measure of how much energy the box itself steals from your reaction.
2. Watch the oxygen levels
Incomplete combustion is the enemy of accuracy. If you're using a bomb calorimeter, the sample is ignited in a pure oxygen environment at high pressure. This forces every single carbon atom to find its oxygen partners. If you're just using a spirit burner and a tripod, your results are going to be "kinda" okay at best—usually $20%$ to $50%$ off the theoretical value.
3. Use the right molar mass
It sounds stupid, but check your periodic table. If you're burning a complex biofuel or a blend, your molar mass ($M$) isn't a simple number. You have to calculate the weighted average based on the fuel's composition.
4. Account for the heat of the spark
In high-precision thermochemistry, even the tiny bit of electricity used to start the fire—the fuse wire or the spark—is subtracted from the total heat measured. It’s a tiny detail, but it’s the difference between a student project and professional-grade data.
Moving Forward with Combustion Data
To master these calculations, you need to be comfortable switching between mass-based energy (like $\text{kJ/g}$) and mole-based energy ($\text{kJ/mol}$).
👉 See also: Finding the Right Keyboard for iPad 10th Generation: Why Most People Overspend
Start by practicing the conversion of $q$ to $\Delta H$ using small sets of data. Once you can consistently remember to flip the sign to negative and divide by the moles of fuel (not moles of water!), you've cleared the biggest hurdle. From there, dive into Hess's Law cycles. They are the most reliable way to predict energy yields for new, experimental fuels where direct measurement is too risky or expensive.
Always double-check your units. If your $c$ is in $\text{J/g}\cdot\text{K}$, your $q$ will be in Joules. But most enthalpy tables are in kiloJoules ($\text{kJ}$). Dividing by $1,000$ is the step everyone forgets right before they submit their work. Don't let that be you.