You're looking at a piece of gold. It’s shiny. It’s heavy. But at the most fundamental level, what makes that gold gold and not a lump of lead or a breath of oxygen? It's a single number. Just one. That’s the atomic number. If you change it, you literally change the identity of the universe. Honestly, it’s the most important digit in chemistry, yet people often trip over how to find it or—worse—confuse it with atomic mass.
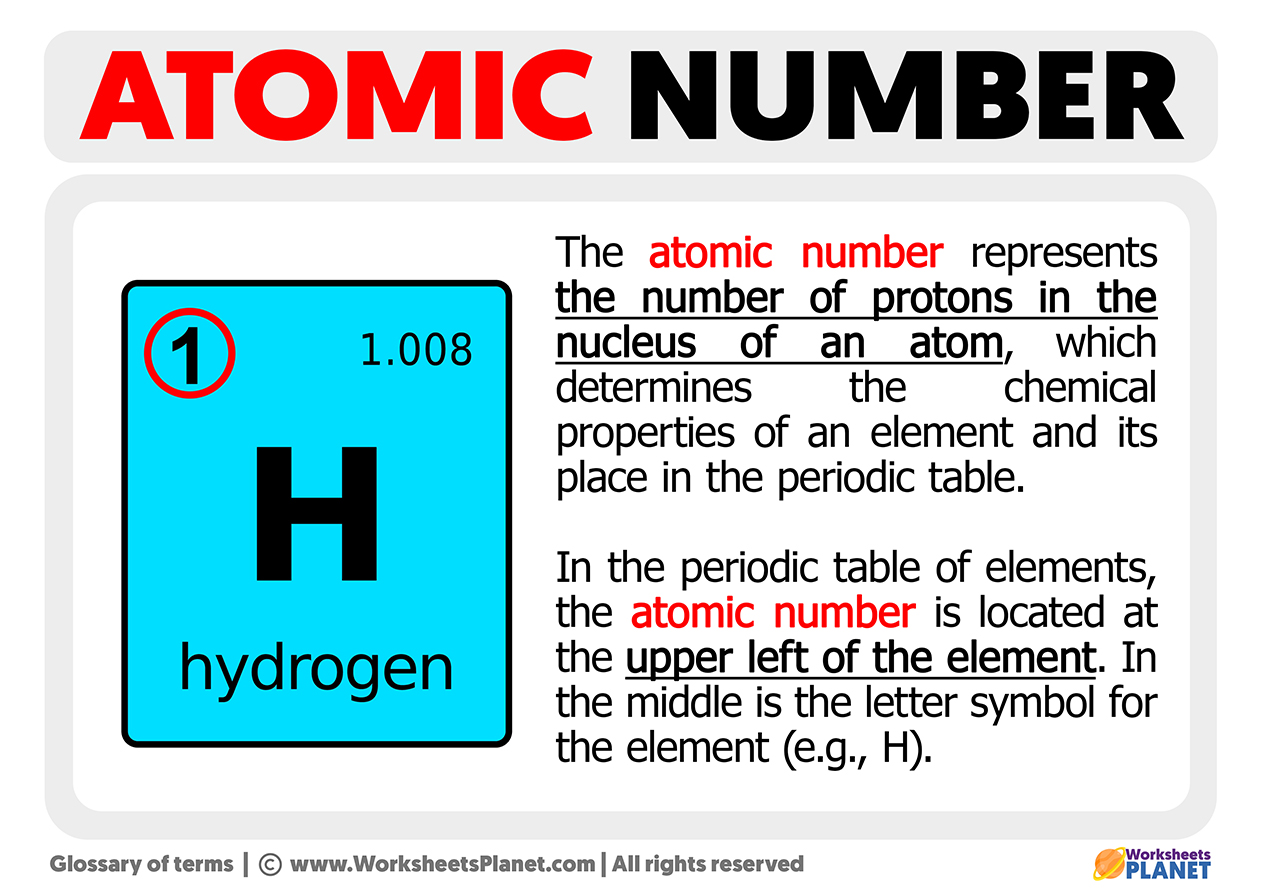
Basically, the atomic number is the ID card of an element. It tells you exactly how many protons are stuffed into the nucleus of an atom. If you have 79 protons, you’re gold. If you lose one and drop to 78, you’re suddenly platinum. It’s that rigid. There's no wiggle room here.
📖 Related: Why the Apple Store W 14th Street Is Still the Best Place to Shop in New York
How to Determine Atomic Number Using the Periodic Table
The easiest way to get this number? Look at a periodic table. Seriously. It’s usually the most prominent whole number in the element's box. Usually, you’ll find it sitting right at the top, above the element symbol.
Take Carbon. You’ll see a "6" hovering over the "C." That 6 is your atomic number. It means every single carbon atom in your body, in your diamond ring, or in the lead of your pencil (which is actually graphite) contains exactly six protons.
Don’t confuse it with the decimal number at the bottom. That’s the atomic mass, which is a weighted average of isotopes. The atomic number is always an integer. You can't have half a proton. Henry Moseley, a brilliant English physicist, was the one who actually figured this out back in 1913. Before him, people just sort of guessed based on weight, which led to all sorts of messy errors. Moseley used X-ray spectroscopy to prove that the atomic number is the real deal. He literally rearranged how we see the building blocks of reality.
Finding Atomic Number Without a Table
What if you don't have a chart handy? Maybe you're in a lab or staring at a physics problem. You can still figure it out if you have a bit of data about the atom’s subatomic particles.
Remember this simple "cheat code":
Protons = Atomic Number.
If a problem tells you an atom has 11 protons, the atomic number is 11. Period. That’s Sodium (Na). It doesn't matter how many neutrons or electrons are floating around; those don't define the element's identity.
The Neutral Atom Trick
In a neutral atom—one that doesn't have a positive or negative charge—the number of electrons equals the number of protons. So, if you’re told a neutral atom has 15 electrons, you know the atomic number is 15. That’s Phosphorus.
But be careful.
Chemistry loves to throw curveballs.
If the atom is an ion, it has gained or lost electrons. A Sodium ion ($Na^+$) still has an atomic number of 11 because it still has 11 protons, but it only has 10 electrons. If you try to use the electron count to find the atomic number for an ion, you're gonna have a bad time. Always look for the protons first.
Using Net Charge to Work Backwards
Sometimes you’ll get a tricky setup where you know the electron count and the net charge.
Let's say you have an ion with 18 electrons and a charge of $-2$.
Since a $-2$ charge means there are two more electrons than protons, you just subtract those two extra electrons.
$18 - 2 = 16$.
The atomic number is 16. That’s Sulfur.
📖 Related: Challenger space shuttle debris: Why we are still finding it 40 years later
The Mass Number Trap
This is where students usually mess up. They see "Mass Number" and "Atomic Number" and think they're interchangeable. They aren't. Not even close.
The mass number is the sum of protons AND neutrons.
If you know the mass number and the number of neutrons, you can determine the atomic number by simple subtraction.
$Mass Number - Neutrons = Atomic Number$.
Imagine an isotope of Carbon called Carbon-14. The "14" is the mass number. We know Carbon-14 has 8 neutrons.
$14 - 8 = 6$.
There it is again. The atomic number is 6.
Why This Number Actually Matters for Real Life
This isn't just academic busywork. Determining the atomic number is how forensic scientists identify unknown substances. It's how astronomers determine what stars are made of by looking at light signatures (spectroscopy).
When a star burns, it’s basically a massive factory for atomic numbers. It starts with Hydrogen (1) and fuses it into Helium (2). As it gets older and hotter, it builds higher and higher numbers—Carbon (6), Oxygen (8), all the way up to Iron (26).
Everything around you—the silicon in your phone, the calcium in your bones—was defined by these numbers being forged in the heart of a dying star. If the atomic number of oxygen were 9 instead of 8, it would be fluorine. You wouldn't be breathing it; it would be eating your lungs from the inside out. One little proton changes everything.
💡 You might also like: Keurig K-Supreme Smart: Why Your Coffee Tastes Better (or Worse) Than Your Neighbor's
Common Misconceptions to Avoid
- "The atomic number can change during a chemical reaction." Nope. Never. Chemical reactions only involve electrons. If the atomic number changes, you’re doing nuclear physics (fusion or fission), not chemistry.
- "Isotopes have different atomic numbers." Actually, isotopes of the same element have the same atomic number but different mass numbers. Carbon-12 and Carbon-14 are both atomic number 6. That's why they're both Carbon.
- "It's always half the atomic mass." This is sort of true for small elements like Helium (mass 4, atomic number 2), but as atoms get bigger, they need way more neutrons to act as "glue" to keep the protons from flying apart. Lead has an atomic number of 82, but its mass is over 207.
Practical Steps to Master Atomic Numbers
If you want to get fast at this, stop trying to memorize the whole table. Nobody does that except for show-offs.
First, get comfortable with the "Big Four": Hydrogen (1), Carbon (6), Nitrogen (7), and Oxygen (8). These make up most of you.
Second, practice the subtraction method. Grab a textbook or a worksheet and find the number of neutrons for various isotopes. Subtract them from the mass. If you can do that, you've mastered the logic behind the math.
Lastly, always check the charge. If a question mentions an "ion," your electron count is a liar. It's trying to trick you. Ignore the electrons and hunt for the protons or the mass/neutron combo.
Your Cheat Sheet for Success
- Look at the Table: It's the big integer at the top of the box.
- Count Protons: Protons are the atomic number.
- Neutral Atoms: Electrons = Atomic Number.
- Math it out: Mass Number - Neutrons = Atomic Number.
- Check the Symbol: Often, the atomic number is written as a subscript to the left of the element symbol ($_{6}C$).
Understanding how to determine atomic number is basically learning the alphabet of the universe. Once you know the numbers, the rest of chemistry starts to actually make sense because you aren't just memorizing names—you're understanding the structural blueprint of matter itself.