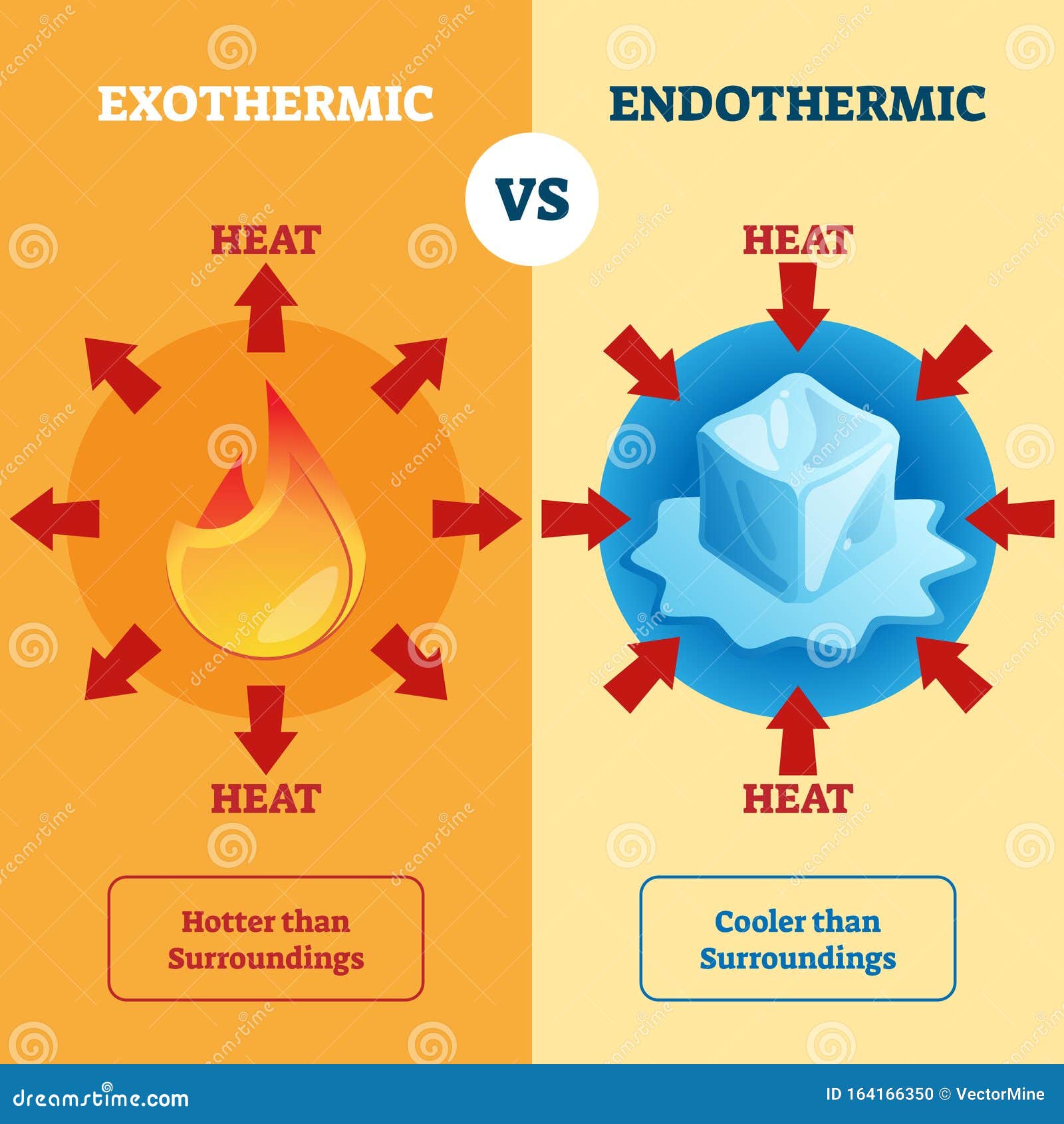
Energy is weird. We can’t see it, yet it dictates every single thing that happens in the universe, from a star collapsing to the way your phone battery heats up while you’re charging it. If you’ve ever sat in a chemistry lab staring at a screen or a textbook, you’ve seen those lines that go up and down like a roller coaster. Those are exothermic and endothermic graphs, or more formally, reaction coordinate diagrams.
Honestly, they look simple. Too simple. And that’s exactly where people mess up.
Most students think it’s just about whether the line ends higher or lower than where it started. While that’s technically true, focusing only on the "start" and "end" is like watching a movie but skipping everything between the opening credits and the finale. You miss the climax. In chemistry, that climax is the activation energy, and if you don't understand it, the graphs won't ever make sense.
Why the Shape of the Curve Actually Matters
Chemistry is basically a cosmic game of "pay to play." You have to put energy in to break bonds before you can get any energy out. This is why a piece of wood doesn't just burst into flames sitting on your desk; it needs a spark.
Let’s talk about the exothermic side of things first. Think about a hand warmer. You crack it, a reaction starts, and suddenly your fingers aren't freezing anymore. In an exothermic graph, the reactants start at a certain energy level. The line then shoots up—that’s the energy you put in—and then drops way down, ending lower than the starting point.
Why? Because the products are more stable than the reactants.
$$\Delta H < 0$$
That little formula above is the math version of saying "I gave away more energy than I took in." When the enthalpy change ($\Delta H$) is negative, the surroundings get hot. It’s a literal release. If you were standing next to that reaction, you’d feel it. But here’s the kicker: even the most violent exothermic reactions have an "up" part of the graph. That’s the activation energy ($E_a$). Even a grenade needs a pin pulled and a strike to go off.
The Struggle of the Endothermic Climb
Endothermic reactions are the high-maintenance friends of the chemical world. They don't give; they take. Constantly.
💡 You might also like: Finding a Safe Windows XP SP3 Download: What Most People Get Wrong
When you look at an endothermic graph, the products end up higher than the reactants. It looks like someone trying to push a boulder up a hill and just... staying there. Photosynthesis is the heavy hitter here. Plants take low-energy water and carbon dioxide and, using the "investment" of sunlight, turn them into high-energy glucose.
The energy doesn't disappear. It’s stored in the bonds.
If you touch an endothermic reaction, like a chemical cold pack used for sports injuries, it feels freezing. People often get this backward. They think "cold" means "no energy." It’s actually the opposite. The reaction is so "hungry" for energy that it’s sucking the heat out of your skin to reach the top of that graph.
The Transition State: The Point of No Return
Right at the peak of those exothermic and endothermic graphs is something called the transition state or the activated complex.
It’s a fleeting, unstable moment.
The old bonds are half-broken, and the new bonds are half-formed. It’s the highest point of potential energy. Think of it like a pencil balancing on its tip. It can’t stay there. It’s going to fall one way or the other. In a successful reaction, it falls toward the products. If there isn't enough energy to reach that peak, the "pencil" just falls back toward the reactants, and nothing happens.
Catalysts change this entire landscape. If you add a catalyst, the peak of the graph gets lower. It’s like finding a tunnel through the mountain instead of climbing over it. The start and end points (the enthalpy) stay exactly the same, but the "barrier to entry" is reduced. This is how the enzymes in your saliva break down starch at body temperature instead of needing a blowtorch to do the job.
Misconceptions That Kill Your Grade
One huge mistake is assuming that all "fast" reactions are exothermic.
Nope.
Speed (kinetics) and energy release (thermodynamics) are two different things. You can have a very slow exothermic reaction—like iron rusting. It releases heat, but it happens so slowly over years that you’d never feel it. Conversely, you can have fast endothermic reactions.
Another one? The idea that "stable" means "low energy." While generally true in these diagrams, stability is relative. On an exothermic graph, the products are more stable relative to the reactants. That’s why the energy dropped. Nature is lazy; it wants to be in the lowest energy state possible.
Breaking Down the Math (Briefly)
You’ll often see these graphs labeled with $H$ for Enthalpy.
- Exothermic: $H_{products} - H_{reactants} = \text{negative value}$
- Endothermic: $H_{products} - H_{reactants} = \text{positive value}$
If you’re looking at a graph and the "tail" is lower than the "head," it’s exothermic. If the "tail" is higher, it’s endothermic. Simple as that.
Real World: Why Should You Care?
This isn't just academic fluff. Our entire global economy is essentially a fight over managing these graphs.
When we burn natural gas (methane) to heat homes, we are utilizing a massive downward slope on an exothermic graph. We want that energy. On the flip side, when we try to create hydrogen fuel through electrolysis, we are fighting an uphill endothermic battle. We have to put electricity in to "climb" the graph and store that energy in hydrogen bonds so we can use it later.
Understanding the "hump" in the middle—the activation energy—is also how we develop better fire retardants or more efficient car engines. If you can control the peak, you control the world.
Actionable Takeaways for Mastering Reaction Graphs
To truly get comfortable with this, don't just look at the pictures. Try these steps:
📖 Related: Does Delete and Report Junk Work? What Really Happens to Those Texts and Emails
- Sketch from memory: Draw an exothermic graph. Now, on the same axes, draw what happens if you add a catalyst (lower the peak). Then, draw the reverse reaction. The reverse of an exothermic reaction is always endothermic.
- Identify the 'System' vs. 'Surroundings': Always ask yourself, "Where is the heat going?" If it’s leaving the test tube and hitting your hand, the graph for the stuff inside the tube is going down (exothermic).
- Label the Transition State: Every time you see a curve, mark the very top as the transition state. Remind yourself that this is the most unstable part of the process where atoms are in mid-rearrangement.
- Check the Enthalpy ($\Delta H$): Practice calculating the difference between the start and end heights. Don't worry about the peak when calculating $ \Delta H $; only the "before" and "after" levels matter for the total energy change.
- Observe daily reactions: Next time you cook an egg, realize you are watching an endothermic process. The egg isn't changing because it wants to; it’s changing because you’re forcing heat into it to climb that energy graph.
By looking at these diagrams as maps of energy flow rather than just lines on a page, the "why" behind chemical behavior becomes much clearer. The universe is just trying to balance its checkbook, and these graphs are the ledger.