Ever wonder why your phone doesn't just die after five minutes, or why those Fourth of July fireworks actually turn that brilliant, searing red? It’s the chemistry. Specifically, it’s the chaotic, reactive world of the left side of the periodic table. We’re talking about examples of alkali metals and alkaline earth metals, two groups of elements that basically run the modern world behind the scenes.
They’re cousins, sorta. But one group is way more "extra" than the other. If you drop a chunk of pure sodium into a pond, it doesn't just sink; it skims across the surface like a frantic water bug before exploding in a hiss of hydrogen gas. That’s an alkali metal for you. They’re desperate to lose an electron. They’re unstable. They’re the rockstars of the elemental world—high energy and prone to dramatic outbursts.
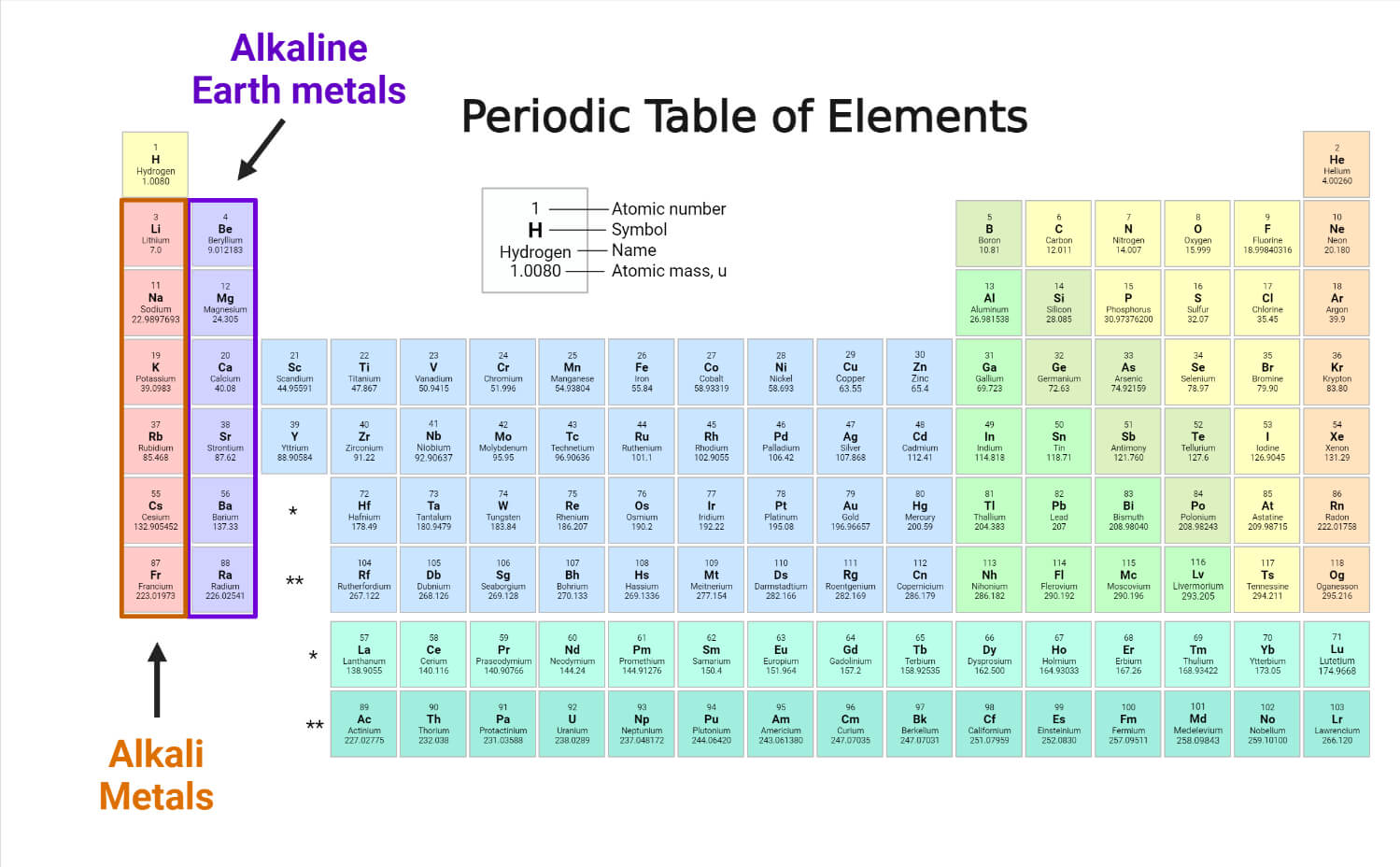
The frantic world of alkali metals
Group 1. That’s where the alkali metals live. Lithium, sodium, potassium, rubidium, cesium, and francium. Forget hydrogen—it’s just hanging out there because it has one electron, but it’s not part of the club. These metals are weirdly soft. You can literally cut a block of pure sodium with a butter knife, and it looks like shiny silver for about three seconds before the air tarnishes it.
Lithium is the one everyone knows now. It’s the heart of your iPhone. It’s the lightest metal, and because it’s so reactive, it’s great at moving energy around. But lithium isn’t just for batteries. Doctors have used lithium carbonate for decades to treat bipolar disorder. It’s a strange bridge between high-tech hardware and the human mind.
Then you have Sodium. Most people think "salt" when they hear sodium, but pure sodium is a nightmare to handle. In its chloride form ($NaCl$), it’s essential for life. It regulates your blood pressure and keeps your nerves firing. Without it, your brain literally couldn't tell your legs to move. It’s the same story with Potassium. You find it in bananas, sure, but in the industrial world, it’s a massive component of fertilizer. We’re basically turning potassium into food.
💡 You might also like: Dokumen pub: What Most People Get Wrong About This Site
The heavier guys, like Cesium, are even more intense. Cesium is used in atomic clocks. It’s so precise that it defines what a "second" actually is. The vibration of a cesium atom is the heartbeat of the GPS system in your car. If cesium stopped working, global navigation would collapse instantly.
Why alkaline earth metals are the "strong, silent type"
Move one column to the right and you hit Group 2. These are the alkaline earth metals. Magnesium, calcium, strontium, barium, and radium. They’re a bit more chill than the alkali metals, but only a bit. They have two electrons in their outer shell instead of one, which makes them slightly less likely to explode if they see a drop of rain, but they’re still plenty reactive.
Calcium is the heavy hitter here. You’ve been told since kindergarten that it builds strong bones, and that’s true. But it’s also the stuff of mountains. Limestone, marble, chalk—it’s all calcium carbonate. When you look at the Great Pyramids or the white cliffs of Dover, you’re looking at the legacy of an alkaline earth metal.
Then there’s Magnesium. It’s incredibly light but surprisingly strong when you mix it with aluminum. You’ll find it in laptop frames and high-end car wheels. It’s also the secret behind that blinding white light in emergency flares. If you’ve ever used a "fire starter" camping tool, that silver rod you scrape sparks off of is often a magnesium alloy.
📖 Related: iPhone 16 Pink Pro Max: What Most People Get Wrong
Comparing the two groups: Not all metals are created equal
Think of it like this: Alkali metals are the "fast" elements. They react instantly. They never exist alone in nature. You will never go for a hike and find a nugget of pure potassium sitting in a creek. It’s always bonded to something else because it’s too "lonely" to stay solo.
Alkaline earth metals are a bit more grounded. They’re harder, they have higher melting points, and they’re way more common in the Earth’s crust as minerals.
- Valence Electrons: Alkali have 1; Alkaline Earth have 2.
- Density: Alkaline earth metals are denser and "tougher."
- Flame Colors: This is the cool part. Lithium burns red, sodium burns a bright "school bus" yellow, and potassium gives off a lilac/purple hue. Meanwhile, strontium (alkaline earth) is responsible for that deep, vivid red in fireworks, and barium gives you the greens.
The weird and the dangerous
We have to talk about Radium. Marie Curie famously discovered it, and it’s a terrifying example of an alkaline earth metal. It’s radioactive. Back in the early 20th century, people thought it was a "health tonic." They put it in toothpaste and painted it on watch dials so they’d glow in the dark. The "Radium Girls," workers who licked their brushes to get a fine point while painting these dials, ended up with horrific bone cancers because the body confuses radium with calcium. Since they’re in the same group, your bones "think" radium is calcium and soak it right up. It’s a tragic lesson in periodic table trends.
Then there’s Francium. It’s the rarest alkali metal. It’s so radioactive and unstable that if you actually managed to get a pound of it in one place, the heat from its own radioactivity would vaporize it instantly. There’s probably less than an ounce of it on the entire planet at any given time.
👉 See also: The Singularity Is Near: Why Ray Kurzweil’s Predictions Still Mess With Our Heads
Real-world impact you can see
If you want to see these elements in action, look at a "Street Light." Those old-school orange ones? Those are sodium vapor lamps. The light is created by passing electricity through—you guessed it—sodium.
Or look at your medicine cabinet. Magnesium hydroxide is what’s in Milk of Magnesia. It neutralizes stomach acid. It’s crazy to think that a metal used to build airplane parts is also something you swallow to fix a stomach ache, but that’s the beauty of chemistry. The form matters. The pure metal is reactive; the ion is essential.
Actionable steps for the curious
If you're trying to wrap your head around these elements for a class or just for fun, don't just memorize the names. Look for them in your daily life.
- Check your multivitamin: Look for calcium and magnesium. Notice how they are often grouped together.
- Check your "Low Sodium" salt: It’s usually potassium chloride. Taste it. It’s slightly metallic and bitter compared to regular salt.
- Watch a video of "Cesium in water": It’s a 10-second lesson in why Group 1 is the most reactive group on the chart.
- Look at your phone's battery label: It’ll say "Li-ion." That’s Lithium. You’re holding a Group 1 alkali metal in your hand right now.
Understanding these groups isn't just about passing a test. It's about realizing that the world isn't just "stuff." It's a precisely organized collection of elements that have very specific personalities. The alkali metals are the energetic, reactive spark plugs of the world, while the alkaline earth metals are the structural backbone of our planet and our bodies.