Energy isn't just a buzzword for your morning coffee. In the world of biology and chemistry, energy is a strict currency that follows the laws of thermodynamics with a ruthlessness that would make a tax auditor blush. When we talk about how life actually works at a cellular level, we are really talking about the dance between two types of reactions: endergonic and exergonic.
Think about it this way. Your body is constantly building things up and tearing things down. It’s a messy, non-stop construction site. If you’ve ever wondered why you need to eat to survive, or why you get hot when you exercise, you’re looking at the visible side effects of these chemical processes. It’s all about the Gibbs free energy.
The Reality of Exergonic Reactions
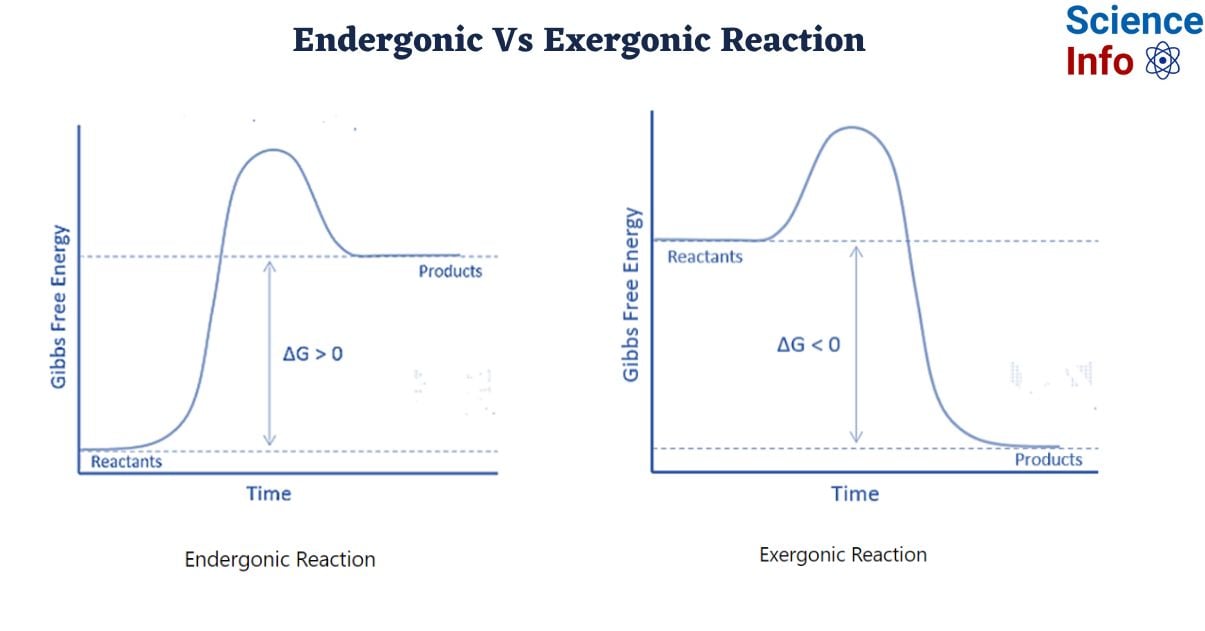
Basically, an exergonic reaction is a "downhill" process. Energy comes out. The system loses free energy, meaning the change in Gibbs free energy ($\Delta G$) is negative. It’s spontaneous, but don't let that word fool you. "Spontaneous" in chemistry doesn't mean it happens fast. It just means it can happen without a constant shove from the outside. Rusting is exergonic. It's also incredibly slow.
The most famous exergonic reaction in your daily life? Cellular respiration.
When you break down glucose, you aren't just making carbon dioxide and water. You’re releasing the energy stored in those chemical bonds. According to the late biochemist Albert Lehninger in his foundational texts, this release is what allows the cell to perform work. Without exergonic reactions providing the "push," nothing else in your body moves.
Imagine a ball sitting at the top of a hill. If you give it a tiny nudge—what scientists call activation energy—it rolls down on its own. That’s exergonic. It releases potential energy as it goes. In your cells, this energy isn't just wasted as heat (though some of it is); it's captured to fuel the stuff that shouldn't be possible.
What People Get Wrong About Endergonic Reactions
Now, an endergonic reaction is the opposite. It’s an "uphill" climb. These reactions require an input of energy to proceed. The products end up with more free energy than the reactants started with. This means $\Delta G$ is positive.
Because they require energy, people often think of endergonic reactions as "inefficient" or "difficult." That’s a mistake. Without endergonic processes, you wouldn't exist. Protein synthesis—the way your body builds muscle, skin, and enzymes—is deeply endergonic. Photosynthesis, the process that literally keeps the planet's food chain intact, is the ultimate endergonic reaction.
Plants take "low energy" molecules like water and carbon dioxide and, using the power of sunlight, force them into "high energy" sugar molecules. It’s like trying to push that ball back up the hill. It won’t happen unless you’re putting in the work.
The Magic of Energy Coupling
Here is the secret sauce. In isolation, an endergonic reaction shouldn't really happen. The universe is lazy; it prefers things to move toward disorder and lower energy (entropy). So, how does your body build a complex protein chain?
It cheats. Or rather, it uses energy coupling.
Biologists like Neil Campbell have detailed how cells pair an exergonic reaction with an endergonic one. The most common "middleman" here is ATP (Adenosine Triphosphate). The breakdown of ATP into ADP is a highly exergonic reaction. It releases a burst of energy. Your cells "tack" that release onto an endergonic process, like moving a mineral across a cell membrane. The energy from the ATP "downhill" slide provides the "upstairs" push for the other reaction.
If the total $\Delta G$ of the two combined reactions is negative, the whole thing moves forward. It’s a brilliant bit of biological accounting.
Why This Matters for Your Health
This isn't just for textbooks. Your metabolism is essentially the sum of these reactions. When you're "burning calories," you are measuring the exergonic breakdown of fuel. If your body can't efficiently couple these with endergonic repair processes, you feel fatigued, your muscles don't recover, and your brain fog sets in.
Consider the "uncoupling" that happens in certain metabolic states. In brown adipose tissue (brown fat), the exergonic flow of electrons in the mitochondria is sometimes uncoupled from the endergonic production of ATP. Instead of making "energy currency," the energy is released entirely as heat. This is why babies, who have more brown fat, can stay warm even when it’s cold—they are literally burning fuel just for the warmth, bypassing the usual storage steps.
🔗 Read more: What Does Delusion Mean? The Reality Most People Get Wrong
The Misconception of "Spontaneity"
One of the biggest hurdles for students and even some health enthusiasts is the word "spontaneous." You’ll hear that exergonic reactions are spontaneous. You might assume that means they happen instantly.
Wrong.
If exergonic reactions were always fast, you would spontaneously combust. You are made of carbon-based molecules that "want" to react with oxygen to form $CO_2$ and water (an exergonic process). The only reason you aren't on fire right now is activation energy. Even downhill reactions need a spark to get started. In your body, enzymes act as the catalysts that lower this "spark" requirement, allowing reactions to happen at body temperature without killing you.
Real-World Examples to Remember
- Exergonic: Mixing baking soda and vinegar. It happens, energy (and gas) is released. Breaking down the food you ate for lunch.
- Endergonic: Charging a phone battery. Building a new strand of DNA before a cell divides.
Complexity and Limitations
While the $\Delta G$ value tells us if a reaction is possible, it doesn't tell us the whole story. Temperature and concentration play massive roles. In a lab, a reaction might be endergonic at room temperature but become exergonic if you crank up the heat. This is why fevers are such a double-edged sword; they speed up chemical reactions to help fight infection, but if they get too high, they start denaturing the very proteins your body worked so hard (through endergonic reactions!) to build.
Furthermore, the "closed system" vs "open system" debate is crucial. The laws of thermodynamics technically apply to closed systems. Humans are open systems. We are constantly exchanging matter and energy with our environment. This allows us to maintain a low-entropy (highly organized) state by constantly increasing the entropy of our surroundings (mostly by pooping and radiating heat).
How to Apply This Knowledge
Understanding the push and pull of energy can change how you view nutrition and exercise.
- Optimize Recovery: Since muscle building is endergonic, it requires an immense amount of "coupled" energy. This is why eating enough—specifically carbohydrates to fuel ATP production—is just as important as protein for growth. You can't build the "upstairs" without the "downhill" fuel.
- Manage Metabolic Heat: If you're overtraining, you might notice your body temperature stays elevated. This is your body struggling with the efficiency of these reactions.
- Respect the Enzyme: Since enzymes control the "activation energy" for these reactions, micronutrients like magnesium and zinc (which act as cofactors) are non-negotiable. Without them, your exergonic reactions stall, and your endergonic "construction" stops.
Basically, life is a constant battle against equilibrium. When a system reaches equilibrium, $\Delta G = 0$. There is no more capacity to do work. In biological terms, that is the literal definition of death. Staying alive means keeping the energy flowing—constantly pushing reactions uphill so they can eventually roll back down.
Next time you feel a burst of energy after a meal, you’re feeling the transition from exergonic breakdown to the endergonic rebuilding of your own tissues. It's a violent, beautiful, and incredibly precise system that keeps you moving.
✨ Don't miss: Hot water bottle for stomach ache: Why this old-school fix actually works
To get a better handle on your own metabolic health, start by tracking your resting heart rate and body temperature over a week. Shifts in these can often signal how well your body is managing its energy coupling. If you're consistently cold or sluggish, your "downhill" exergonic processes might not be providing enough "push" for your daily needs.