Life is uphill. Honestly, if you look at the physics of it, being alive is a constant struggle against the natural tendency of the universe to fall apart into a mess of high entropy. This is where the endergonic reaction definition biology students often memorize comes into play, but most textbooks make it sound way more boring than it actually is.
Energy isn't just "there." It has to be shoved into place.
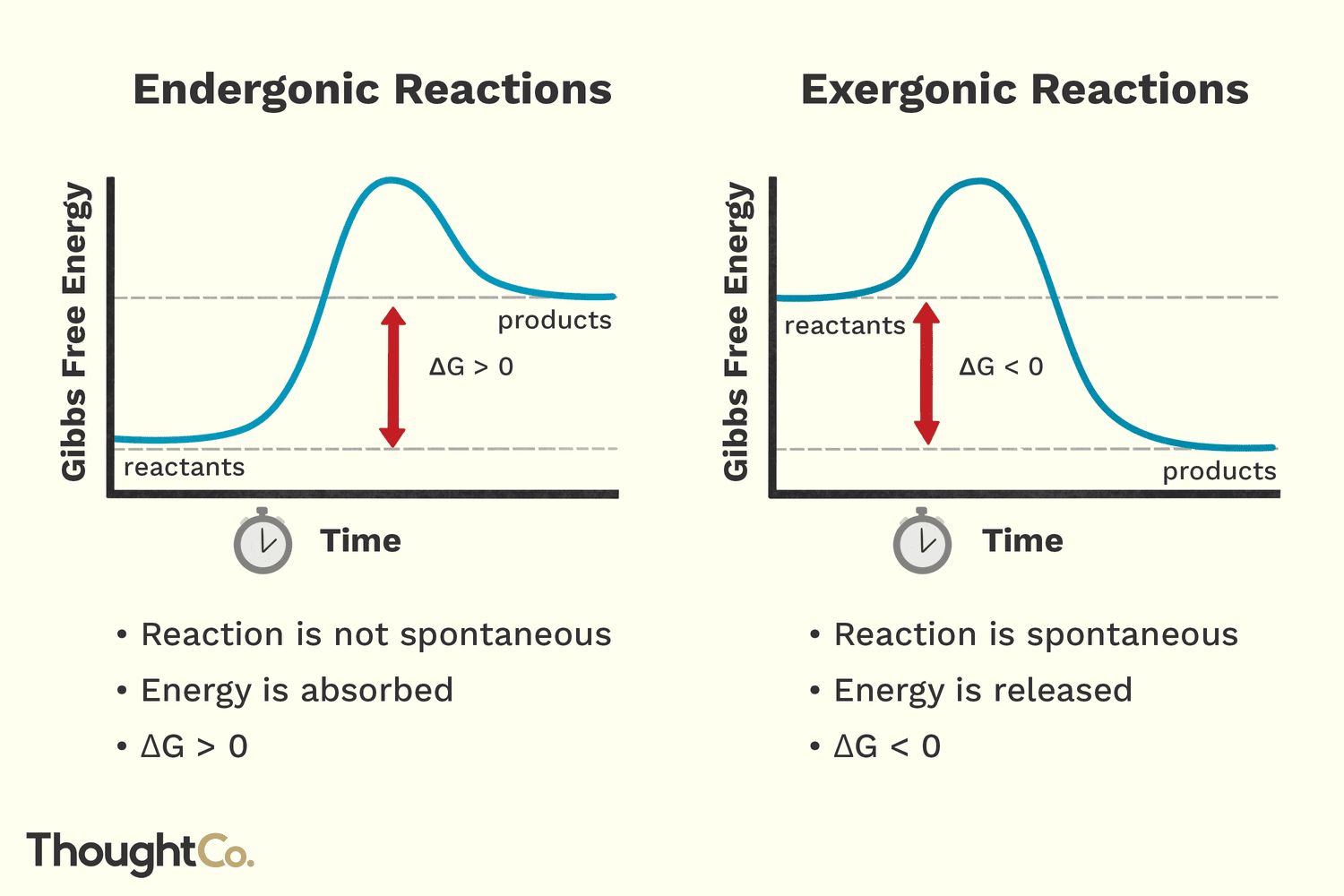
Think of an endergonic reaction like a massive boulder you’re trying to roll up a steep hill. It doesn’t want to go up there. You have to put in physical work to make it happen. In biological terms, an endergonic reaction is any chemical process that requires a net input of free energy to proceed. If you don't feed it, it stops. This is the opposite of exergonic reactions, which release energy like a boulder crashing back down that same hill.
The Math of Making Things Happen
Biologists use a specific term called Gibbs Free Energy, or $\Delta G$. For an endergonic reaction, the change in free energy is positive ($\Delta G > 0$). This means the products of the reaction actually contain more energy than the starting materials.
It’s non-spontaneous. That’s the "official" word. It means the reaction won't just happen because two molecules bumped into each other in the dark. You need a spark, a push, or a massive amount of ATP to force those bonds to form. Without this "uphill" climb, you wouldn't have DNA, you wouldn't have muscles, and you certainly wouldn't be able to read this screen.
Photosynthesis: The Ultimate Energy Thief
Plants are the masters of this. They take carbon dioxide and water—two very low-energy, boring molecules—and use the literal power of a star to force them into complex, energy-dense glucose.
This is the quintessential endergonic reaction definition biology example.
✨ Don't miss: Why Sometimes You Just Need a Hug: The Real Science of Physical Touch
Sunlight provides the "work" needed to bridge the energy gap. If the sun went out, the endergonic process of photosynthesis would cease immediately. The glucose molecules are essentially tiny batteries that have stored up all that solar effort. When you eat a salad, you're just stealing the energy that the plant worked so hard to push uphill.
Why Your Body Loves ATP
So, how does a human body do this? We don't have solar panels. We use a trick called reaction coupling.
Basically, your cells take a very "happy" reaction—one that releases a ton of energy (exergonic)—and pin it to a "sad" reaction that needs energy (endergonic). This is usually where ATP (adenosine triphosphate) comes in. When ATP loses a phosphate group to become ADP, it releases energy. Your cell "grabs" that energy and immediately uses it to build a protein or move a muscle fiber.
It’s like using a falling weight to pull a bucket of water up a well. The falling weight is the exergonic part; the rising water is the endergonic part.
Building Muscle and the Cost of Growth
Anabolism is just a fancy word for "building stuff." When you go to the gym and lift weights, your body starts synthesizing new protein strands (actin and myosin) to repair and grow your muscles. This is a massive endergonic undertaking.
You need:
🔗 Read more: Can I overdose on vitamin d? The reality of supplement toxicity
- Amino acids (the bricks).
- Ribosomes (the construction workers).
- Heaps of ATP (the electricity for the power tools).
This is why you get hungry after a workout. Your body has spent its energy reserves forcing small molecules into large, complex structures. Creating order from chaos is expensive.
Misconceptions About Heat and Speed
A common mistake is thinking endergonic reactions are always cold or that they are always slow. Not true. While they do "absorb" energy, that doesn't always mean they feel cold to the touch like an ice pack (though many endothermic reactions do).
Also, enzymes.
Enzymes don't change the fact that a reaction is endergonic. They don't magically make the reaction "free." What they do is lower the activation energy—the initial "oomph" needed to get things started. But at the end of the day, you still have to pay the energy tax. If the products have more energy than the reactants, that energy has to come from somewhere. No exceptions. Physics is a strict accountant.
The Nuance of Equilibrium
In a closed system, endergonic reactions would eventually just stop. But biological systems are "open." We are constantly breathing, eating, and excreting. This prevents our chemical reactions from reaching equilibrium. If your body's chemistry reached equilibrium, you’d be dead. Literally. Equilibrium in biology is just another word for "no more work can be done."
We keep the concentrations of reactants high and products low to keep the "uphill" flow moving. It's a dynamic, shifting balance that requires constant monitoring by your nervous and endocrine systems.
💡 You might also like: What Does DM Mean in a Cough Syrup: The Truth About Dextromethorphan
Real-World Implications for Health
Understanding the endergonic reaction definition biology helps explain metabolic disorders. When someone has a "slow metabolism," it often means their body's efficiency in coupling these energy-releasing and energy-consuming reactions is slightly off.
If you can't efficiently drive endergonic processes, your body can't repair tissues, build hormones, or maintain its internal temperature. Chronic fatigue syndrome and certain mitochondrial diseases often boil down to a failure in managing these "uphill" energy requirements.
Moving Beyond the Textbook
Most people stop learning about this after their high school biology final. That’s a mistake. Understanding how your body hoards and spends energy changes how you look at nutrition and exercise. It isn't just about calories; it’s about the work of staying complex.
Next time you eat a meal, don't just think of it as fuel. Think of it as the raw "push" your body needs to keep performing the thousands of endergonic reactions that keep your heart beating and your brain thinking. You are a walking, talking defiance of entropy.
Practical Steps for Energy Management
- Prioritize Protein Intake: Since protein synthesis is a major endergonic process, you need the right raw materials (amino acids) and enough caloric "push" to make the synthesis happen.
- Optimize Mitochondrial Health: Your mitochondria are the factories where ATP is made to power these reactions. CoQ10, magnesium, and regular Zone 2 cardio help keep these factories efficient.
- Understand Recovery: Sleep is when your body does its most intense endergonic "rebuilding" work. If you cut sleep, you're cutting the time your body has to roll those boulders back up the hill.
- Monitor Glucose: Since glucose is the primary starting point for creating the ATP that drives these reactions, maintaining stable blood sugar ensures a steady "energy currency" flow.
Focus on the "why" behind the energy. If you're feeling sluggish, it’s likely that your body's ability to fund its endergonic requirements is being taxed. Look at your micronutrient intake—specifically B vitamins and magnesium—as these act as the "lubricant" for the enzymes that manage energy coupling. Stop viewing your metabolism as a simple furnace and start viewing it as a complex construction site that requires constant, active investment.