Honestly, the world of Alzheimer’s treatment moves so fast it's kind of hard to keep up. One day we’re talking about "miracle drugs," and the next, there’s a massive debate about whether the NHS will even touch them. If you’ve been following the donanemab news october 2025 cycle, you know it's a bit of a rollercoaster.
We are finally seeing the "real world" data. It’s not just clinical trials anymore. People are actually sitting in infusion chairs.
The big headline this month? The European Commission finally gave donanemab (you might know it by the brand name Kisunla) the green light. But, like most things in medicine, there's a catch. Or three.
The State of Donanemab News October 2025
So, what's actually happening on the ground?
In the U.S., the FDA recently updated the label for Kisunla. They’ve added a modified titration schedule. Basically, doctors are now starting patients on a lower, more gradual dose to try and stop the brain from swelling or bleeding—what the experts call ARIA. It’s a huge deal because ARIA has been the "boogeyman" of these amyloid-clearing drugs.
Across the pond, the situation is... complicated.
✨ Don't miss: Why Meditation for Emotional Numbness is Harder (and Better) Than You Think
In late September, the European Commission authorized the drug for adults with early-stage Alzheimer’s. This was a huge win for Eli Lilly. However, the UK's spending watchdog, NICE, has basically said "thanks, but no thanks" for the NHS. They’re citing the massive cost of the drug and the intensive monitoring required. It’s a frustrating setback if you’re a family in Great Britain hoping for public coverage.
Why the "Stop and Go" Dosing Matters
One of the coolest things about donanemab—and something people often get wrong—is that you don’t take it forever.
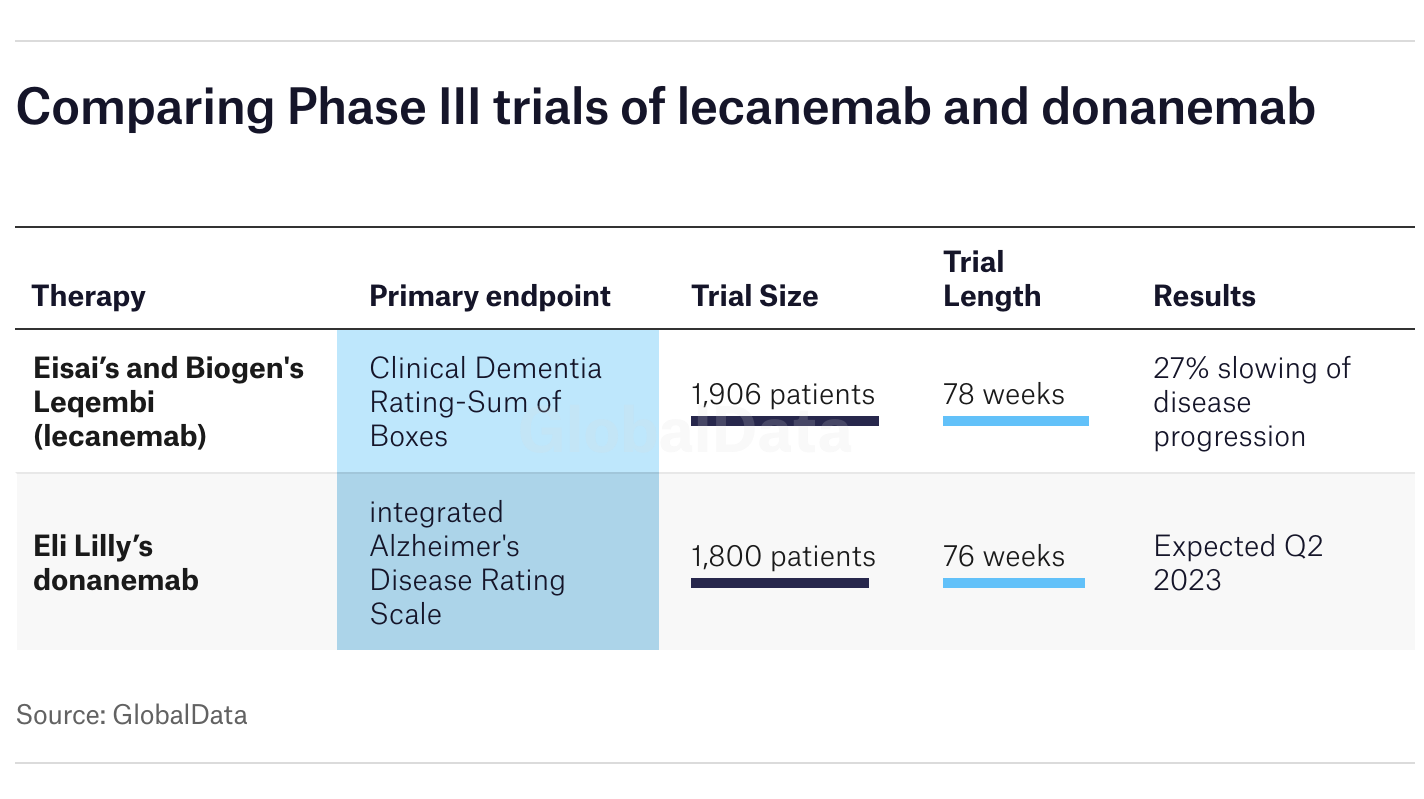
Unlike its rival lecanemab (Leqembi), which is usually an indefinite treatment, donanemab is designed to be stopped once the amyloid plaques are gone. New data from the TRAILBLAZER-ALZ 2 long-term extension study, presented earlier this year and still being dissected this October, shows that more than 75% of patients cleared their plaques within 76 weeks.
Once those plaques are gone, you stop.
The data suggests the benefits keep "rolling" for up to three years, even after you've stopped the infusions. It's sort of like a deep clean for the brain. You do it, you finish it, and you hope the results last.
🔗 Read more: Images of Grief and Loss: Why We Look When It Hurts
The Tau Factor: Who Actually Benefits?
Not everyone is a candidate. This is the nuance that gets lost in the "breaking news" style of reporting.
The latest analysis confirms that donanemab works best for people with "low-to-medium" levels of tau protein. Tau is another protein that tangles up in the brain. If you have too much tau already, the damage might be too far gone for an amyloid-clearing drug to do much.
Because of this, many clinics are now requiring a tau PET scan before they even think about prescribing it. It’s a bottleneck. These scans are expensive. They aren't available everywhere.
Safety, ARIA, and the Genetic Lottery
We have to talk about the side effects. It’s not all sunshine.
The risk of ARIA (Amyloid-Related Imaging Abnormalities) is significantly higher for people who carry two copies of the APOE4 gene. In fact, European regulators have restricted the drug’s use specifically to those with one or no copies of that gene.
💡 You might also like: Why the Ginger and Lemon Shot Actually Works (And Why It Might Not)
- ARIA-E: This is swelling (edema).
- ARIA-H: These are tiny bleeds (microhemorrhages).
Most cases are mild. You wouldn't even know you had it without an MRI. But in rare cases, it's fatal. Three deaths in the original trials were linked to these complications. This is why the October 2025 updates on dosing titration are so critical—they are trying to make the drug safer for the average person.
Comparing the Big Two: Donanemab vs. Lecanemab
If you're sitting in a neurologist's office today, you're likely choosing between these two. Here's the raw truth on how they stack up based on the latest 2025 meta-analysis:
- Dosing Frequency: Lecanemab is every two weeks. Donanemab is every four weeks. For a lot of families, once a month is just easier to manage.
- Duration: As mentioned, donanemab has a finish line. Lecanemab is generally continuous.
- Effectiveness: Some recent studies suggest donanemab might be slightly more effective at clearing plaques quickly, but it also carries a slightly higher risk of ARIA compared to lecanemab.
- Cost: Both are expensive, roughly $32,000 a year in the U.S. before insurance.
What This Means for You Right Now
If you or a loved one are looking into this, the donanemab news october 2025 landscape tells us a few things clearly.
First, get your genetics checked. You need to know your APOE4 status. Second, don't wait until symptoms are "obvious." These drugs are for the very early stages. If someone can't remember how to use a microwave or get home from the store, they might already be past the window where donanemab helps.
Also, check your insurance. While Medicare in the U.S. is covering it (if you're in a registry), private insurers like Aetna and UnitedHealthcare have very specific "precertification" rules as of October 2025. They want proof of amyloid via a PET scan or a lumbar puncture. No proof, no pay.
Actionable Steps to Take
- Request a Biomarker Test: Ask your doctor for a p-tau217 blood test. It’s a newer, easier way to see if there’s a high probability of Alzheimer’s before jumping to expensive PET scans.
- Check the Registry: In the U.S., coverage often requires participation in a registry like ALZ-NET. Make sure your clinic is actually set up to do this.
- Evaluate the "Tau Burden": If you can afford it or if it's covered, get a tau PET scan. It’ll tell you if donanemab is actually going to work for your specific brain chemistry.
- Monitor the NHS Appeal: If you're in the UK, keep an eye on the NICE final decision scheduled for late 2025. There's a chance they might negotiate a lower price with Eli Lilly.
The reality of Alzheimer’s treatment is changing. We’ve moved from "there's nothing we can do" to "there's something we can do, but it's complicated and expensive." That’s progress, even if it feels slow.
For the most part, the goal now is simply buying time. If a drug can give a person an extra 6 to 12 months of living independently, that’s hundreds of more dinners, birthdays, and conversations. For many, that’s worth the risk.