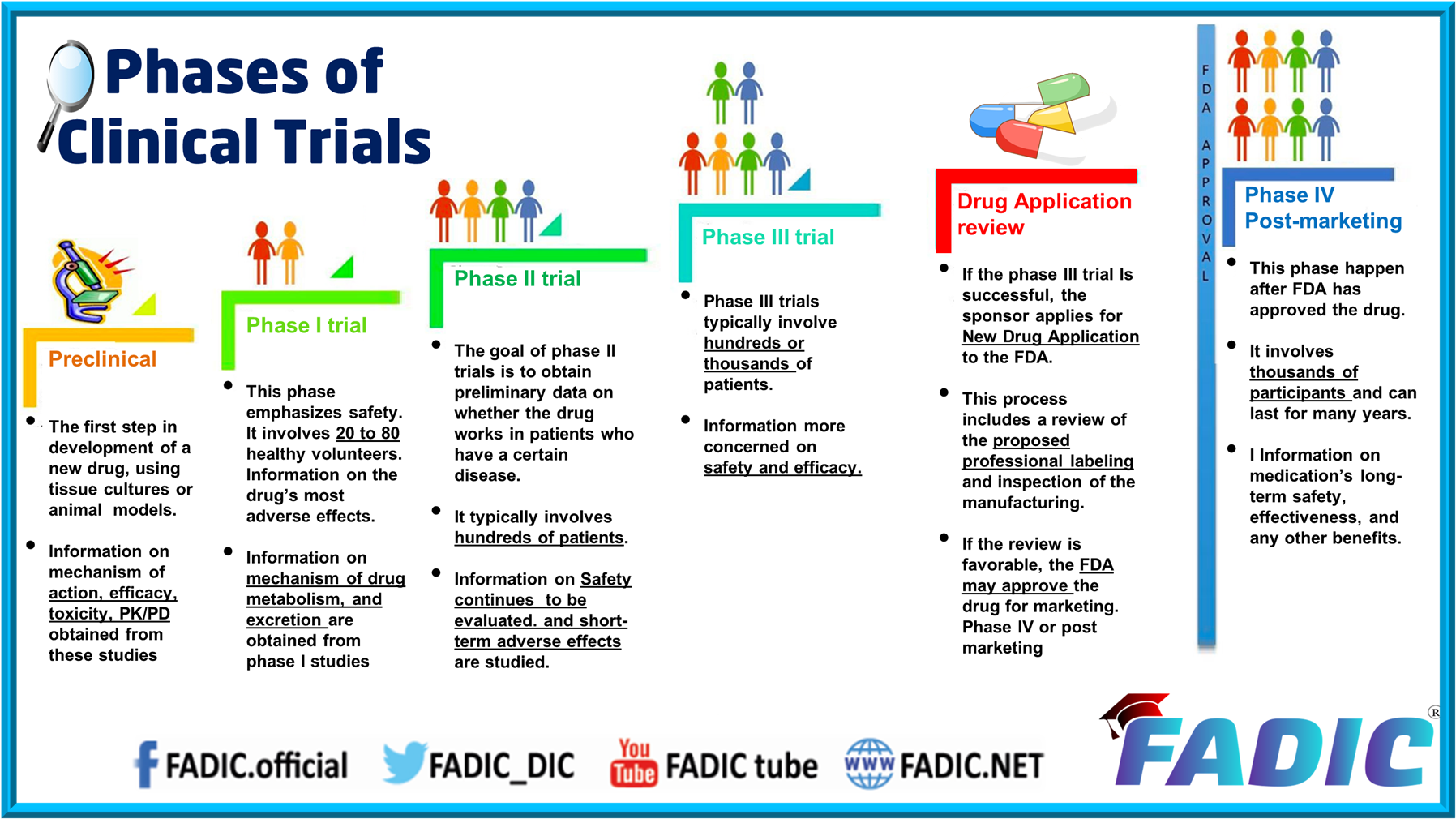
You'd think, with all the billions poured into biotech, that the data would be the easy part. It isn't. Honestly, most people outside the industry assume that once a patient swallows a pill or gets an injection, the "data" just magically appears on a clean spreadsheet ready for the FDA. If only. Clinical trial data services are actually the messy, complicated engine room of the entire medical world, and right now, that engine is undergoing a massive, somewhat chaotic overhaul.
Most trials fail. Not always because the drug is bad, but because the data is a wreck. Think about it. You have dozens of sites globally, hundreds of nurses entering notes, and thousands of patients using clunky apps or, god forbid, paper diaries. Clinical trial data services are the specialized bridge that tries to turn that noise into something a regulator won't laugh at. It’s about more than just storage; it’s about survival in a market where a single "dirty" data point can delay a drug launch by years.
The Messy Reality of Data Management
Data management isn't just "IT for doctors." It’s closer to forensic accounting mixed with high-stakes logistics. Back in the day—and I mean like ten years ago—clinical data management was mostly about Electronic Data Capture (EDC). You’d build a digital form, someone would type in a blood pressure reading, and that was that.
Now? It’s a nightmare of volume. We’re talking wearable sensors streaming heart rates every second, genomic sequencing files that are absolutely massive, and "Real World Data" (RWD) pulled from insurance claims or electronic health records. Most traditional clinical trial data services are drowning because their old systems weren't built for "streaming" life; they were built for "snapshots" of it.
Take the rise of Decentralized Clinical Trials (DCTs). When COVID-19 hit, the industry had to pivot or die. Suddenly, the "site" wasn't a hospital in Boston; it was a patient's living room in rural Ohio. This shift forced data services to become hyper-mobile. If a patient’s wearable device stops syncing, does the data service have a protocol to flag it in ten minutes, or does it wait three weeks for a manual audit? That’s the difference between a successful Phase III and a total loss of $100 million.
Why Quality Is Actually Getting Harder to Guarantee
Quality is a funny word in this business. Everyone claims they have it. But "clean data" is becoming an endangered species. According to recent industry benchmarks from the Tufts Center for the Study of Drug Development, the complexity of clinical trial protocols has increased by nearly 70% over the last decade. More endpoints. More procedures. More data points per patient.
When you add more layers, you add more room for human error.
A big misconception is that AI just fixes this. It doesn't. Not yet, anyway. While some clinical trial data services use machine learning to spot outliers—like a patient whose weight stays exactly the same for six months, which is statistically impossible—AI is only as good as the metadata. If the person at the clinic didn't label the blood sample correctly, the most expensive algorithm in the world is just going to process garbage.
The Human Element.
✨ Don't miss: Social Media Number Search: How to Find Profiles Without Getting Scammed
We still need "Data Managers" who actually understand biology. You need someone who looks at a lab result and thinks, "Wait, that potassium level is physically impossible for a living human," rather than a script that just checks if the box is filled.
The Tech Stack: It’s Not Just One Tool
If a vendor tells you they have an "all-in-one" platform that solves everything, they’re lying to you. Or they’re sell-side marketing people who’ve never seen a CRF (Case Report Form) in their lives. Real-world clinical trial data services use a "best-of-breed" approach.
- EDC (Electronic Data Capture): The backbone. This is where the core trial data lives.
- eCOA/ePRO: Electronic Clinical Outcome Assessments. This is the app on the patient's phone where they report how they feel.
- IRT (Interactive Response Technology): This manages the actual drug supply. You can't have data if the patient doesn't have the meds.
- Datalakes: Because the EDC can't hold the massive files from an MRI or a genetic panel.
The real magic—and where most companies fail—is the integration. Getting these four things to talk to each other in real-time is the holy grail. Most of the time, they’re just "bolted" together with manual file transfers that happen on Tuesday nights. It’s clunky. It’s slow. And it’s why your favorite biotech stock probably just missed its primary endpoint.
What Most People Miss: The Regulatory "Shadow"
Regulations like 21 CFR Part 11 aren't just suggestions. They are the law. In the US, the FDA requires a "gold standard" audit trail. Every single click, every change of a value, every "oops, I meant 120 over 80, not 210 over 80" must be tracked.
I’ve seen trials get stalled not because the drug was dangerous, but because the clinical trial data services provider couldn't prove who changed a data point or why. This is the "traceability" nightmare. If you can't reconstruct the trial from the data logs, the trial basically didn't happen in the eyes of the regulators.
European trials have it even tougher with GDPR. Mapping patient privacy onto a global data stream is a headache that requires specialized legal-tech expertise. You can't just send "Patient 004's" data to a server in Virginia without a mountain of paperwork and encryption protocols that would make a bank jealous.
Cost vs. Value: The Race to the Bottom
There’s a dangerous trend right now toward commoditization. Big Pharma is trying to squeeze costs, so they look for the cheapest clinical trial data services. Bad idea.
Saving $50k on a data management contract is great until you realize your database lock is delayed by two months because the offshore team didn't understand the protocol's specific "skip logic." A two-month delay on a blockbuster drug can cost a company millions in lost patent life. It’s the ultimate example of being "penny wise and pound foolish."
Experienced sponsors are moving toward "Functional Service Provider" (FSP) models. Instead of hiring a giant company to do everything, they hire a specific team of data experts who basically become part of their own company. It’s more intimate. It’s more accountable. And honestly, it’s the only way to handle the weird, specific quirks of rare disease trials or oncology where the data is incredibly dense.
The Rise of "Risk-Based" Everything
We used to check 100% of the data. Every single number was verified against a source document. It was called Source Data Verification (SDV), and it was incredibly boring and expensive.
Now, the industry has moved to Risk-Based Monitoring (RBM). Basically, we use stats to figure out which sites are likely to mess up. If Site A has perfect records and Site B has weirdly fast entry times and high "adverse event" rates, the clinical trial data services team focuses all their energy on Site B.
It’s smarter. It’s faster. But it requires a level of data visualization that most legacy providers just don't have. You need dashboards that update hourly, not monthly. You need to see the "smoke" before there’s a "fire."
Small Biotechs are in Trouble
If you’re a small "one-asset" biotech, the data service landscape is terrifying. You don't have the leverage of Pfizer. You're often a low priority for the big CROs (Contract Research Organizations).
Small teams need to be scrappy. They need clinical trial data services that are "agile"—a word that’s overused but actually means something here. It means being able to change a database mid-study because the FDA suddenly decided they want to see a different lab value. If your data provider takes six weeks to implement a "change order," you’re cooked.
Actionable Steps for Navigating Data Services
Look, if you’re actually in the trenches of a trial, stop looking at the shiny UI of the software. That’s just the paint. You need to look at the engine.
- Audit the Audit Trail: Ask to see how the system handles "query resolution." If it looks like an Excel sheet from 1997, run away.
- Integration is King: Don't ask if they integrate; ask how. If the answer involves "manual CSV uploads," they aren't ready for 2026.
- Demand Real-Time Access: You should be able to see your "enrollment vs. data entry" lag at 2 AM on a Sunday. If you have to wait for a "weekly report," your data service is a bottleneck, not a partner.
- Check the Specialized Experience: Does the team actually know your therapeutic area? Data for a cardiovascular trial is totally different from data for a psychiatric study. One is all hard numbers; the other is all subjective scales. You don't want a "generalist" managing your endpoints.
- Focus on the "Close-Out": Everyone is excited at the start. Nobody talks about the "Database Lock." That’s where the pain is. Ask a provider for their median time from "last patient, last visit" to "database lock." If it’s more than 30 days, they’re slow.
The future of medicine isn't just about the molecule; it's about the math. We're entering an era of "In Silico" trials and digital twins, but none of that happens without the foundation of rock-solid, verifiable, and highly organized data. The providers who win won't be the ones with the most features; they'll be the ones who can actually guarantee that when the FDA asks a question, the answer is only one click away.
Don't settle for a data vendor. Find a data partner. There’s a massive difference between a company that just hosts your data and one that actually protects your trial's integrity. In this game, the data is the drug. Without the proof, you just have a very expensive chemical.
Focus on the architecture first. The insights will follow. If you get the data services right, you’re not just checking boxes; you’re actually getting medicine to the people who need it faster. And at the end of the day, that’s the only metric that matters.
Everything else is just noise.