You’ve probably seen the word "sulfites" on a bottle of wine or a bag of dried apricots. It usually sits there in a tiny font, sounding vaguely like a preservative you should be worried about. But if we’re talking about the actual chemical formula of sulfite, we have to move past the grocery store label and look at the actual geometry of the thing.
The sulfite ion is basically a sulfur atom that grabbed three oxygen atoms and decided it wasn't done yet, so it snatched two extra electrons from the neighborhood.
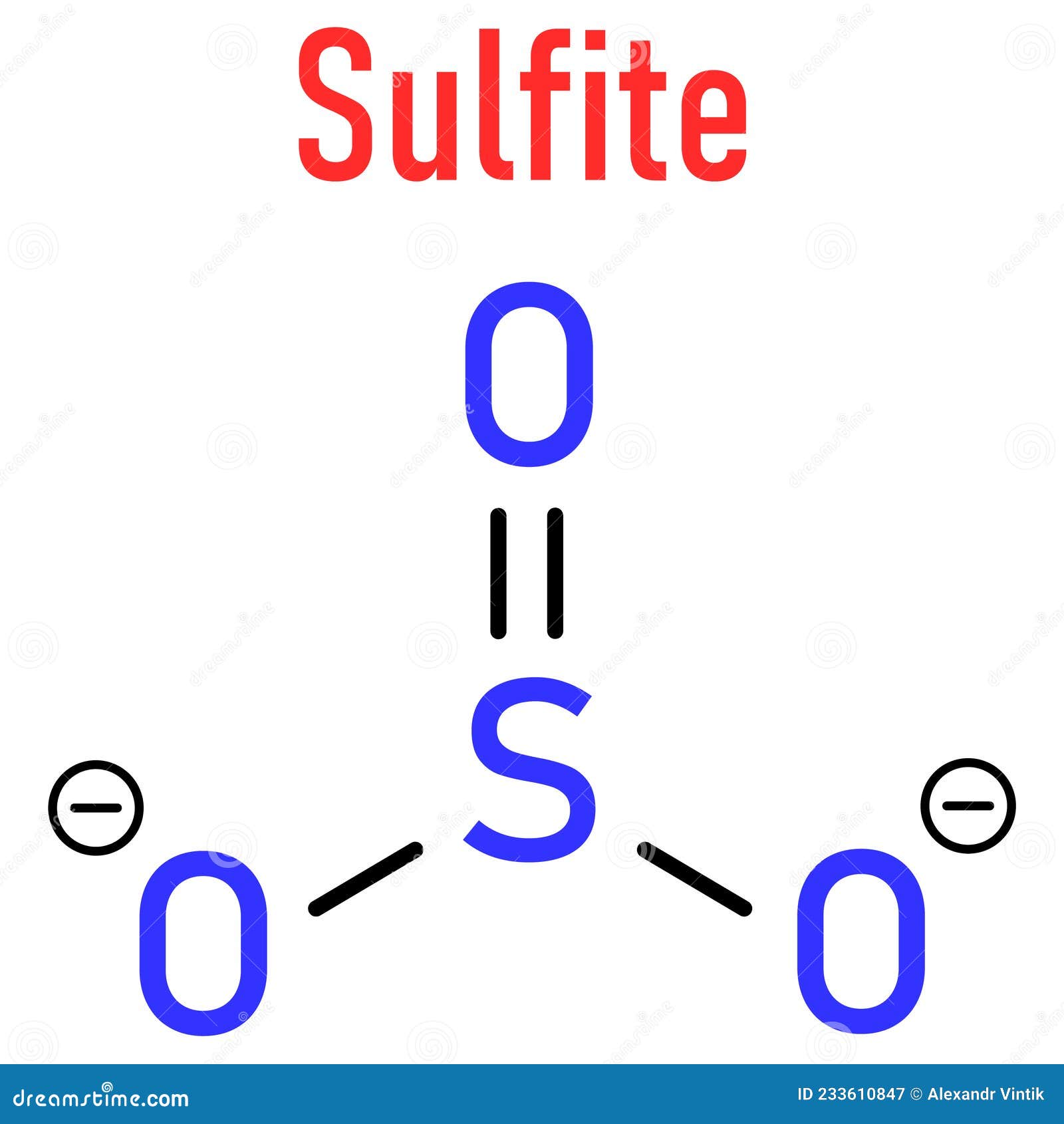
Its chemical signature is $SO_{3}^{2-}$. Simple, right? Well, sort of.
The Reality of the Chemical Formula of Sulfite
Let’s get the technicals out of the way. When we write $SO_{3}^{2-}$, we’re describing a polyatomic ion. It carries a -2 charge. This charge is the reason you rarely find "sulfite" just hanging out by itself in nature like a lonely traveler. It’s almost always paired with something else, usually a metal like sodium or potassium, to balance the scales.
In its most common lab form, you’re looking at sodium sulfite, which is $Na_{2}SO_{3}$.
See what happened there? The two positive sodium ions ($Na^{+}$) cancel out that -2 charge on the sulfite. It’s a chemical handshake. If you change the partner, you change the properties, but the core chemical formula of sulfite remains that specific arrangement of one sulfur and three oxygens.
Why the Geometry is Weird
Sulfur is in Group 16 of the periodic table. It’s got six valence electrons. When it forms the sulfite ion, it uses some of those to bond with oxygen, but it keeps a "lone pair" of electrons for itself.
Imagine a pyramid with a tripod base. The three oxygen atoms form the legs, and the sulfur sits at the top. But on the very top of the sulfur, there’s an invisible cloud of electrons—that lone pair—pushing everything else down. Chemists call this "trigonal pyramidal" geometry. It’s not flat. It’s not symmetrical in the way a carbonate ion ($CO_{3}^{2-}$) is. It’s lopsided.
This lopsidedness matters. It’s why sulfites are so reactive. They are "reducing agents," which is a fancy way of saying they love to steal oxygen from other things or get oxidized themselves. In a bottle of Riesling, that $SO_{3}^{2-}$ is basically a bodyguard. It sees oxygen coming in to ruin the party and intercepts it, turning into sulfate ($SO_{4}^{2-}$) so the wine doesn't turn into vinegar.
👉 See also: The Truth About Every Casio Piano Keyboard 88 Keys: Why Pros Actually Use Them
Where People Get Confused: Sulfite vs. Sulfate vs. Sulfide
Honestly, the names are a nightmare. If you’re a student or just someone trying to read a technical data sheet, it’s easy to mix them up.
- Sulfide is just $S^{2-}$. No oxygen. It smells like rotten eggs.
- Sulfite is our hero today, $SO_{3}^{2-}$.
- Sulfate is $SO_{4}^{2-}$. It’s the "completed" version. It’s stable. It’s what you find in Epsom salts or drywall.
The "ite" ending in the chemical formula of sulfite tells you it has fewer oxygens than the "ate" version. It’s the middle child of the sulfur-oxygen family. Not as bare as sulfide, not as saturated as sulfate.
Real World Behavior and Lewis Structures
If you were to draw the Lewis structure—and many people do because it’s a classic chemistry homework problem—you’d realize that the sulfur atom in sulfite actually has an "expanded octet."
Most atoms want eight electrons. Sulfur is a bit of a glutton. It can handle ten or even twelve because it has access to d-orbitals. In the most stable resonance structure of the sulfite ion, you have one double bond with an oxygen and two single bonds.
Wait. Resonance?
Yeah. The double bond doesn't stay in one place. It flicks between the three oxygen atoms faster than you can blink. This delocalization of electrons makes the ion more stable than it would be otherwise. If you’re looking at a diagram and see dotted lines, that’s just the chemist's way of saying "the double bond is everywhere at once."
Why Your Body Cares About $SO_{3}^{2-}$
We can’t talk about the chemical formula of sulfite without touching on biology. For about 1% of the population, these ions are a problem.
When sulfites enter an acidic environment—like your stomach—they can release sulfur dioxide gas ($SO_{2}$). For most of us, an enzyme called sulfite oxidase handles this instantly. It’s a molybdenum-dependent enzyme that turns the sulfite into sulfate, which we then pee out.
✨ Don't miss: iPhone 15 size in inches: What Apple’s Specs Don't Tell You About the Feel
But if you lack enough of that enzyme?
Then you get the "sulfite sensitivity" symptoms: wheezing, hives, or a nasty headache. It’s not a true allergy in the IgE sense, but it’s a real metabolic roadblock. This is why the FDA requires a "contains sulfites" label if the concentration is over 10 parts per million.
The Food Industry's Best Friend
Despite the health concerns for a small minority, the industry loves the $SO_{3}^{2-}$ ion. It’s a miracle worker.
- It stops "enzymatic browning." You know how sliced apples turn brown? Sulfites stop that.
- It kills wild yeast. In winemaking, you want the specific yeast you added to do the work, not some random fungus from the vineyard.
- It bleaches flour and starches.
The Chemistry of Acid Rain (The Dark Side)
When we burn coal or oil, we release sulfur. This eventually forms sulfur dioxide, which can react with water in the atmosphere to create sulfurous acid ($H_{2}SO_{3}$).
This acid is basically the "protonated" version of the chemical formula of sulfite.
$H_{2}SO_{3} \rightleftharpoons H^{+} + HSO_{3}^{-} \rightleftharpoons 2H^{+} + SO_{3}^{2-}$
It’s a weak acid, but it’s enough to eat away at limestone statues and change the pH of freshwater lakes. When you see a gargoyle on a cathedral in Europe that looks like its face is melting off, you’re looking at the long-term effects of the sulfite/sulfate cycle in the atmosphere.
How to Test for It in a Lab
If you’re stuck in a lab and need to prove you have $SO_{3}^{2-}$ in a solution, there’s a classic trick. You add a dilute acid. If it starts smelling like a struck match, that’s $SO_{2}$ gas escaping.
🔗 Read more: Finding Your Way to the Apple Store Freehold Mall Freehold NJ: Tips From a Local
Another way? Barium chloride.
If you add $BaCl_{2}$ to a solution with sulfite, you get a white precipitate of barium sulfite ($BaSO_{3}$).
But wait! Barium sulfate is also a white precipitate. How do you tell them apart?
You add hydrochloric acid. Barium sulfite will dissolve in the acid; barium sulfate won't. It’s a simple "yes/no" test that chemists have used for over a century to distinguish between these two very similar-looking ions.
Surprising Nuances: The Bisulfite Ion
We can't just talk about $SO_{3}^{2-}$ without mentioning its cousin, $HSO_{3}^{-}$.
This is the bisulfite ion (or hydrogen sulfite). It’s what happens when the sulfite ion catches just one hydrogen atom. In many practical applications, like the "bisulfite sequencing" used in modern genetics to study DNA methylation, this version of the formula is actually the star of the show.
In the lab, these two exist in an equilibrium that depends entirely on the pH. If the environment is very basic, you get sulfite. If it’s slightly more acidic, you get bisulfite. It’s a sliding scale.
Actionable Insights for Handling Sulfites
If you are dealing with sulfites—whether in a chemistry lab, a home brewery, or just trying to manage a sensitivity—here are the hard facts to keep in mind:
- Check the pH: Sulfites are most effective as antimicrobial agents at lower pH levels because they convert into molecular $SO_{2}$, which can penetrate cell walls.
- Oxidation is Inevitable: If you leave a solution of sodium sulfite open to the air, it will slowly turn into sodium sulfate. Always keep your containers tightly sealed.
- Neutralization: if you have a spill, hydrogen peroxide ($H_{2}O_{2}$) is a common way to neutralize sulfites by oxidizing them immediately to the much safer sulfate form.
- Temperature Matters: The solubility of sulfite salts changes drastically with temperature. Sodium sulfite is actually less soluble in very hot water than in lukewarm water—a weird quirk of its crystalline structure.
Understanding the chemical formula of sulfite isn't just about passing a test. It’s about understanding how a tiny group of four atoms can preserve your food, dissolve a statue, or trigger a respiratory response. It’s a lopsided, electron-hungry little ion that punches way above its weight class in the world of inorganic chemistry.
For anyone looking to dive deeper into inorganic salts, your next step should be researching the solubility product constants ($K_{sp}$) of various sulfite salts. This will show you exactly how these ions behave when they meet metal ions in groundwater or industrial waste. You might also look into the Winkler Method, which, while usually for oxygen, demonstrates the redox power that sulfur-oxygen ions bring to the table in analytical chemistry.