You’ve probably heard the hype for years. A single shot. A permanent fix. The end of genetic disease. It sounds like science fiction, doesn’t it? But as we kick off 2026, the reality of cell and gene therapy news is a lot messier—and way more interesting—than the "miracle cure" headlines suggest.
Honestly, the industry just went through a bit of a reality check.
Last year was supposed to be the victory lap for gene therapy. Instead, we saw Pfizer pull the plug on its FDA-approved hemophilia B treatment, Beqvez, in early 2025. Why? Because literally zero patients took it. It turns out that even when you have a $3.5 million "cure," people are hesitant. They’re worried about long-term safety, the crazy high price tags, and the fact that you usually only get one shot at this. If it doesn't work, your immune system might block you from ever trying a similar therapy again.
But don't think for a second that things are slowing down.
On January 11, 2026, the FDA basically rewrote the rulebook. They announced a new, flexible approach to manufacturing and clinical controls specifically for these living medicines. They finally admitted that you can’t regulate a bag of custom-edited cells the same way you regulate a bottle of Tylenol. This shift is huge. It’s designed to stop the "manufacturing bottleneck" that has killed so many promising drugs before they even reached a patient.
The CRISPR Milestone Nobody Expected
If you follow cell and gene therapy news, you know Casgevy was the big star of 2024. It was the first-ever CRISPR drug approved for sickle cell disease. Fast forward to today, and the numbers are finally in. In 2025, Casgevy cleared over $100 million in revenue. That’s more than 60 people who have effectively had their DNA "re-coded" to stop the excruciating pain of sickle cell.
It’s working.
📖 Related: Why the 45 degree angle bench is the missing link for your upper chest
Vertex Pharmaceuticals just confirmed that they are pushing for global filings in the first half of 2026 to treat children as young as five. Think about that. A five-year-old could get a treatment today that prevents a lifetime of organ damage.
But CRISPR isn’t just for rare blood disorders anymore. The focus is shifting to "common" diseases. We’re seeing early data on CRISPR therapies for high cholesterol and cardiovascular disease. Instead of taking a pill every morning for the rest of your life, you might eventually just get one infusion that "silences" the gene responsible for your high LDL.
What Most People Get Wrong About "The Cure"
There is a massive misconception that these therapies are like an "undo" button. They aren't.
Take Hemgenix, the gene therapy for hemophilia B. It’s a breakthrough, sure. But as experts like Dr. Benjamin Samelson-Jones at CHOP have pointed out, it doesn’t take the risk down to zero. It mostly moves a patient from "severe" hemophilia to "mild" hemophilia. You still have to be careful. You still have to monitor your health.
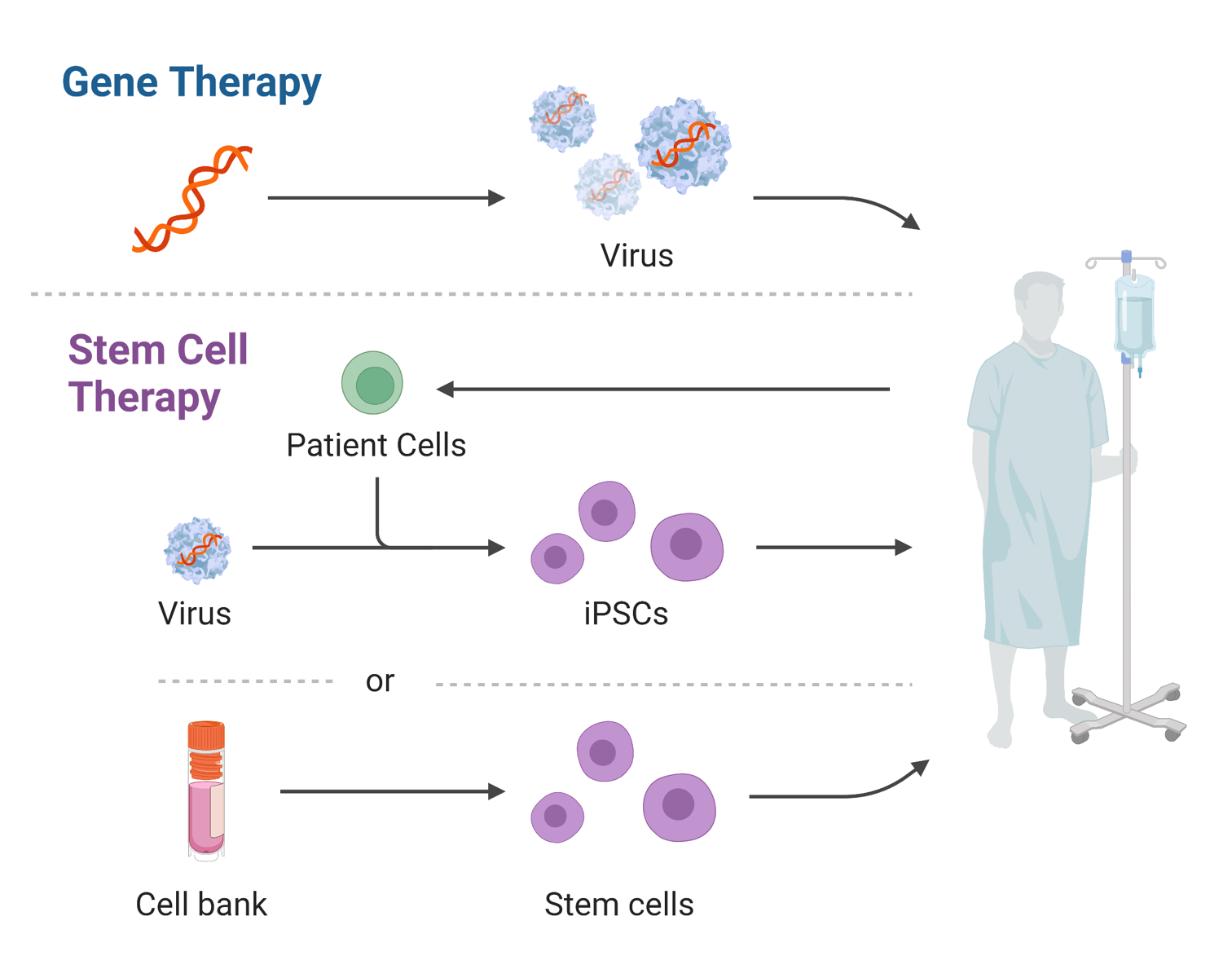
The industry is also grappling with "the one-and-done" problem. Most current gene therapies use a virus (AAV) to deliver the new genetic code. Once your body sees that virus, it develops antibodies. If the therapy wears off in ten years—which we’ve seen happen in some trials—you can’t just go back for a "booster."
This is why 2026 is becoming the year of non-viral delivery.
👉 See also: The Truth Behind RFK Autism Destroys Families Claims and the Science of Neurodiversity
Companies are racing to find ways to get genes into cells using lipid nanoparticles (the stuff in COVID vaccines) or even tiny "envelopes" made of synthetic material. If they crack this, gene therapy becomes repeatable. That changes everything.
Real Breakthroughs You Might Have Missed
While everyone was looking at sickle cell, some wild stuff happened in the background:
- Zevaskyn got approved in late 2025. It’s a therapy for "Butterfly Children" (RDEB) whose skin is so fragile it tears like tissue paper. They take the patient’s skin cells, fix the DNA, grow them into sheets, and surgically graft them back on. It’s actually healing chronic wounds that have stayed open for years.
- Encelto changed the game for vision loss. It’s a tiny implant that’s put into the eye to continuously pump out a protein that keeps the retina from dying. It’s the first "encapsulated cell therapy" for MacTel.
- Papzimeos finally gave an option to people with RRP—a condition where tumors grow in the airway, forcing some patients to have dozens of surgeries just to breathe.
The Business of Hope (and Why It’s Shaking Up)
Let’s talk money, because it’s the elephant in the room. The cell and gene therapy news cycle is often dominated by these multi-million dollar price tags.
We’re seeing a split in the market. The big players like Novartis and Vertex are doubling down on "blockbuster" potential, while smaller startups are struggling to find funding. In 2025, private funding for gene therapy actually dipped. Investors are tired of hearing about "potential"; they want to see patients actually getting treated and insurance companies actually paying.
The market is projected to hit $33.5 billion by the end of this year. But that growth is lopsided. North America still holds over 50% of the market because our reimbursement systems—while messy—are actually starting to figure out how to pay for these drugs over time.
Where We Go From Here
If you’re a patient, a caregiver, or just someone interested in the future of medicine, here is what you need to keep an eye on over the next 12 months.
✨ Don't miss: Medicine Ball Set With Rack: What Your Home Gym Is Actually Missing
1. Watch the 5-11 age group.
The FDA review of CRISPR for younger children in early 2026 will be a bellwether for how aggressive the agency wants to be with gene editing in pediatric populations.
2. Follow the "Autoimmune Pivot."
The coolest thing happening right now isn't in rare disease—it’s in Lupus. CAR-T cells, which were originally made to kill blood cancer, are being tested to "reboot" the immune systems of people with severe Lupus (SLE). The early data from late 2025 was stunning. We might be looking at a way to actually stop autoimmune diseases rather than just suppressing them with steroids.
3. Pay attention to the "Redo" tech.
Look for news about "Re-dosing" or "Non-viral vectors." If a company proves they can give a second dose of a gene therapy, their stock—and the hope for that disease—will skyrocket.
The "miracle" phase of gene therapy is over. We’re in the "workhorse" phase now. It’s about manufacturing, insurance codes, and making sure these treatments actually reach the people who need them. It’s less flashy, but it’s a lot more meaningful.
To stay ahead of these developments, keep a close watch on the FDA's CBER (Center for Biologics Evaluation and Research) updates and the quarterly clinical trial data from companies like CRISPR Therapeutics and Bluebird Bio. The next year will determine if these therapies remain a luxury for the few or a standard of care for the many.
Focus on the results of the "Pivotal" trials—Phase 3 is where the real truth comes out. If a therapy is in Phase 3 right now, 2026 is likely the year it either changes the world or becomes another cautionary tale.